Question
Question: $K_1$, $K_2$ and $K_3$ are the equilibrium constants of the following reactions (I), (II) and (III) ...
K1, K2 and K3 are the equilibrium constants of the following reactions (I), (II) and (III) respectively
I) N2+2O2⇌2NO2 II) 2NO2⇌N2+2O2 III) NO2⇌21N2+O2 The correct relation from the following is
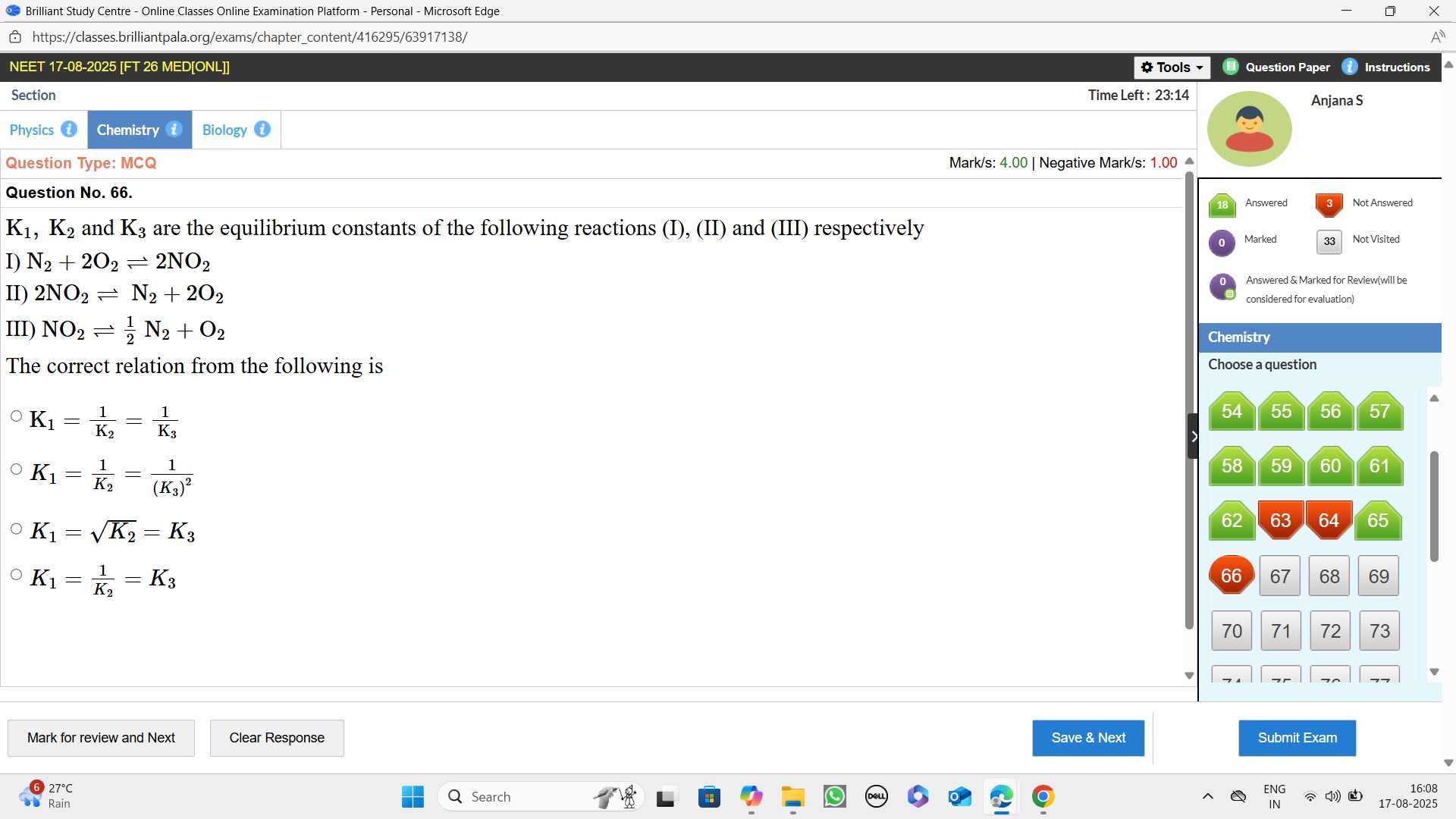
K1=K21=K31
K1=K21=(K3)21
K1=K2=K3
K1=K21=K3
K1=K21=(K3)21
Solution
To determine the correct relation between the equilibrium constants K1, K2, and K3 for the given reactions, we will write down the expression for each constant and then establish the relationships.
The given reactions are: I) N2+2O2⇌2NO2 II) 2NO2⇌N2+2O2 III) NO2⇌21N2+O2
The equilibrium constant expressions are: For Reaction I: K1=[N2][O2]2[NO2]2 For Reaction II: K2=[NO2]2[N2][O2]2 For Reaction III: K3=[NO2][N2]1/2[O2]
1. Relationship between K1 and K2:
Reaction II is the reverse of Reaction I. When a reaction is reversed, its equilibrium constant becomes the reciprocal of the original equilibrium constant. Therefore, K2=K11, which implies K1=K21.
2. Relationship between K1 and K3:
Let's manipulate Reaction III to obtain Reaction I. Reaction III: NO2⇌21N2+O2 (with equilibrium constant K3)
First, reverse Reaction III: 21N2+O2⇌NO2 When a reaction is reversed, its equilibrium constant becomes the reciprocal. So, the equilibrium constant for this reversed reaction is K31.
Next, multiply the reversed reaction by 2: 2×(21N2+O2⇌NO2) This gives: N2+2O2⇌2NO2, which is Reaction I. When a reaction is multiplied by a factor 'n', its equilibrium constant is raised to the power 'n'. Here, n=2. So, the equilibrium constant for Reaction I (K1) will be (K31)2. K1=(K3)21
Combining the relationships:
We have found two relationships:
- K1=K21
- K1=(K3)21
Therefore, the correct combined relation is K1=K21=(K3)21.
