Question
Question: Match the columns Column - I | Column - II ---|--- A) HClO₄ | p) Strong base B) HNO₂ | q) Strong ac...
Match the columns
| Column - I | Column - II |
|---|---|
| A) HClO₄ | p) Strong base |
| B) HNO₂ | q) Strong acid |
| C) NH₂⁻ | r) Weak base |
| D) HSO₄⁻ | s) Weak acid |
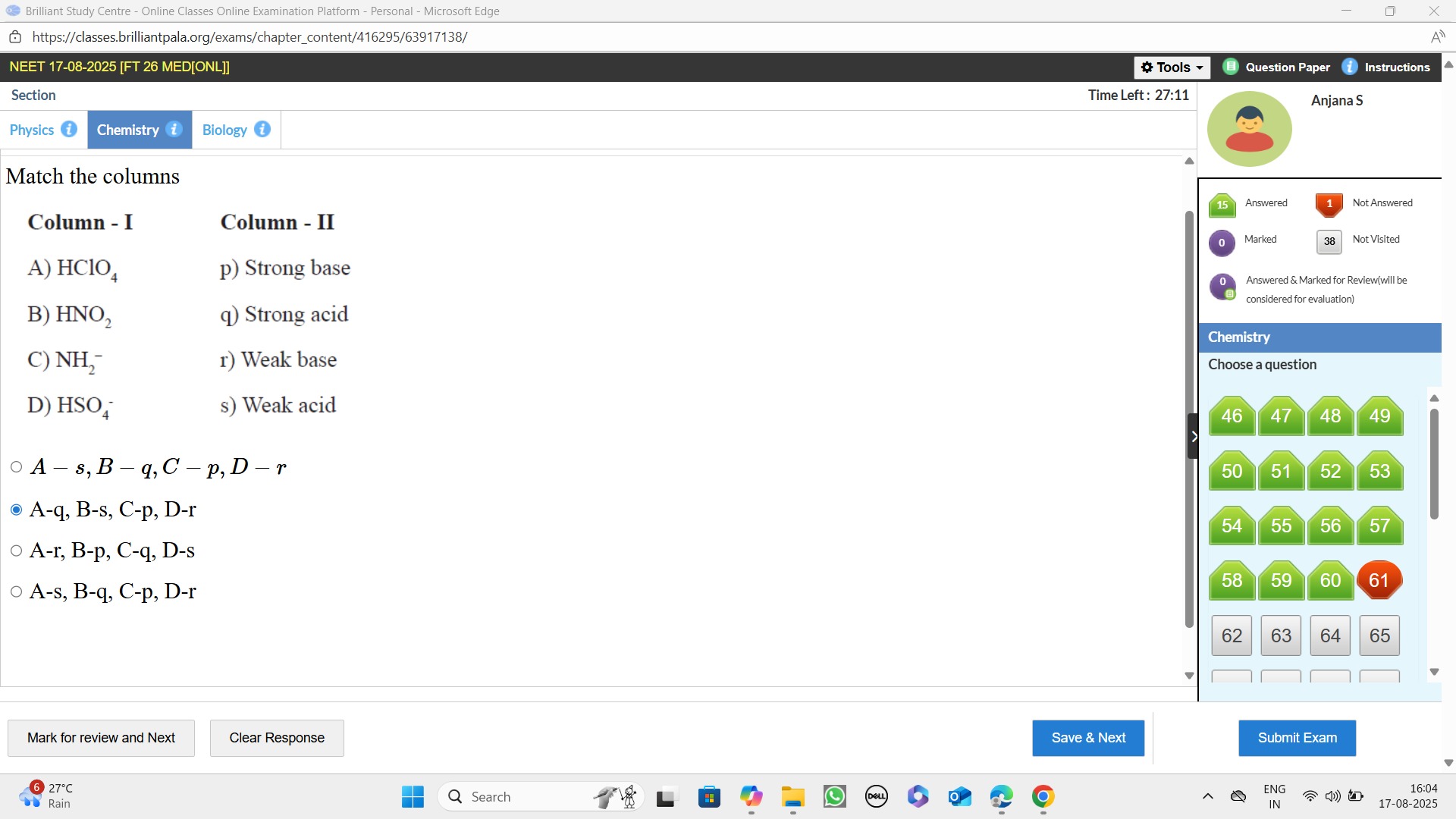
A-s, B-q, C - p, D - r
A-q, B-s, C-p, D-r
A-r, B-p, C-q, D-s
A-s, B-q, C-p, D-r
A-q, B-s, C-p, D-r
Solution
The question asks us to match the given chemical species in Column I with their properties (acid/base strength) in Column II.
Let's analyze each species:
A) HClO₄ (Perchloric acid)
- HClO₄ is an oxyacid. The strength of oxyacids increases with the number of oxygen atoms not bonded to hydrogen. In HClO₄, there are three non-protonated oxygen atoms (H-O-ClO₃).
- Oxyacids with two or more non-protonated oxygen atoms are generally strong acids. HClO₄ is one of the strongest known acids.
- Therefore, HClO₄ is a strong acid.
- Matches with q.
B) HNO₂ (Nitrous acid)
- HNO₂ is also an oxyacid. Its structure is H-O-N=O. It has one non-protonated oxygen atom.
- Oxyacids with zero or one non-protonated oxygen atoms are generally weak acids.
- Therefore, HNO₂ is a weak acid.
- Matches with s.
C) NH₂⁻ (Amide ion)
- The amide ion (NH₂⁻) is the conjugate base of ammonia (NH₃). Ammonia (NH₃) is a weak base.
- However, NH₂⁻ is formed by the deprotonation of NH₃ (NH₃ → NH₂⁻ + H⁺). This is not the usual way to think about it. More accurately, NH₂⁻ is an extremely strong base because it readily accepts a proton from even very weak acids (like water) to form ammonia and hydroxide ions (NH₂⁻ + H₂O → NH₃ + OH⁻). This reaction proceeds almost to completion, indicating NH₂⁻ is a much stronger base than OH⁻.
- Therefore, NH₂⁻ is a strong base.
- Matches with p.
D) HSO₄⁻ (Hydrogen sulfate ion / Bisulfate ion)
- HSO₄⁻ is an amphoteric species, meaning it can act as both an acid and a base.
- As an acid: HSO₄⁻ ⇌ H⁺ + SO₄²⁻. The acid dissociation constant (Kₐ₂) for this reaction is 1.2 × 10⁻², which corresponds to a pKₐ₂ of approximately 1.92. Since pKₐ₂ > 0, HSO₄⁻ is a weak acid.
- As a base: HSO₄⁻ + H⁺ ⇌ H₂SO₄. Sulfuric acid (H₂SO₄) is a very strong acid in its first dissociation (H₂SO₄ → H⁺ + HSO₄⁻). The conjugate base of a very strong acid is a very weak base. Thus, HSO₄⁻ is a very weak base.
- In aqueous solutions, the acidic nature of HSO₄⁻ (its ability to donate a proton) is more prominent and commonly discussed than its basic nature (its ability to accept a proton to reform H₂SO₄, which would immediately dissociate). Therefore, it is primarily classified as a weak acid.
- Matches with s.
Combining the matches: A) HClO₄ → q (Strong acid) B) HNO₂ → s (Weak acid) C) NH₂⁻ → p (Strong base) D) HSO₄⁻ → s (Weak acid)
Now let's check the given options:
- A-s, B-q, C - p, D - r (Incorrect)
- A-q, B-s, C-p, D-r (Incorrect for D)
- A-r, B-p, C-q, D-s (Incorrect)
- A-s, B-q, C-p, D-r (Duplicate of first option)
There appears to be a slight mismatch in the provided options, especially for D. However, if we are forced to choose the "best fit" or if there's an assumption that HSO₄⁻ is considered a weak base in the context of the question (as it is the conjugate base of a strong acid), let's re-evaluate. While HSO₄⁻ is a weak acid (pKₐ₂ = 1.92), it is also the conjugate base of the first dissociation of H₂SO₄ (a strong acid), making it a very weak base. If the option 'r' (weak base) is intended for HSO₄⁻, then Option 2: A-q, B-s, C-p, D-r would be the closest match. Given the typical classification, HSO₄⁻ is more commonly referred to as a weak acid due to its measurable dissociation.
However, if we strictly follow the common understanding and pKa values, D should be 's' (weak acid). Since none of the options perfectly align with D-s, there might be an error in the question or options. Assuming the question intends to classify HSO₄⁻ as a weak base, as it is the conjugate base of a strong acid, then option 2 becomes plausible. Let's consider the most definitive matches: A-q, B-s, C-p. These three are consistent in option 2.
Final determination based on common understanding and best fit among given options: A) HClO₄ - Strong acid (q) B) HNO₂ - Weak acid (s) C) NH₂⁻ - Strong base (p) D) HSO₄⁻ - While a weak acid, it is also a very weak base (conjugate base of strong acid). If 'r' is the intended match, then option 2 is the closest.
Let's assume the question considers HSO₄⁻ as a weak base in this context, as it's a common point of discussion for amphoteric species.
