Question
Question: The order of stability of boron trihalide is...
The order of stability of boron trihalide is
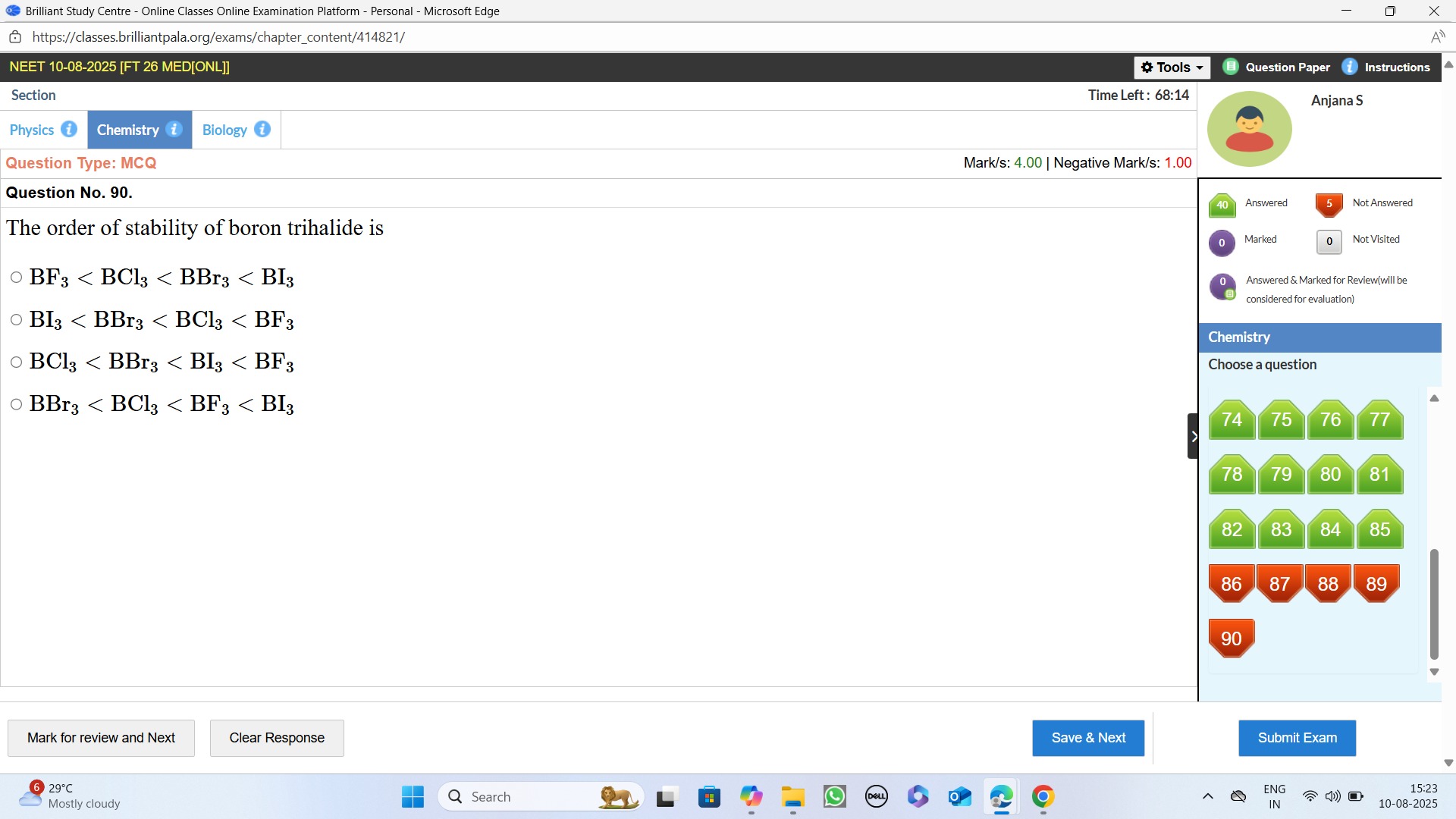
BF3 < BCl3 < BBr3 < BI3
BI3 < BBr3 < BCl3 < BF3
BCl3 < BBr3 < BI3 < BF3
BBr3 < BCl3 < BF3 < BI3
BI3 < BBr3 < BCl3 < BF3
Solution
The stability of boron trihalides (BX3) is primarily determined by the extent of pπ-pπ backbonding from the halogen atom (X) to the boron atom (B).
-
Backbonding: Boron in BX3 compounds is sp2 hybridized and has a vacant 2p orbital. Halogen atoms have lone pairs of electrons in their p-orbitals. These lone pairs can be donated into the vacant 2p orbital of boron, forming a partial π-bond. This phenomenon is called backbonding.
-
Effectiveness of Backbonding: The effectiveness of backbonding depends on the size and energy match between the orbitals involved.
- In BF3, the overlap is between B(2p) and F(2p) orbitals. This 2p-2p overlap is very effective due to the similar size and energy of the orbitals. This strong backbonding significantly reduces the electron deficiency of boron.
- As we move down the group from fluorine to iodine, the size of the halogen's p-orbital increases (Cl: 3p, Br: 4p, I: 5p). The overlap between boron's 2p orbital and the halogen's np orbital becomes progressively less effective due to increasing size and energy mismatch.
- Therefore, the extent of backbonding decreases in the order: BF3>BCl3>BBr3>BI3.
-
Stability and Backbonding: Stronger backbonding leads to greater delocalization of electron density and a more stable molecule, as it reduces the electron deficiency of boron and imparts partial double bond character to the B-X bond.
- Since BF3 has the most effective backbonding, it is the most stable among the boron trihalides.
- BI3 has the least effective backbonding, making it the least stable.
-
Order of Stability: Based on the extent of backbonding, the order of stability is:
BF3>BCl3>BBr3>BI3
In increasing order of stability, this is:
BI3<BBr3<BCl3<BF3
