Question
Question: Natural rubber or raw rubber consists of basic material latex which is a dispersion of isoprene. Dur...
Natural rubber or raw rubber consists of basic material latex which is a dispersion of isoprene. During the treatment, this isoprene forms a high molecular weight polymer of isoprene. Natural rubber can be obtained from five hundred different species of plants.
Consider the following properties of rubber,
1.The tensile strength of the vulcanized rubber is almost ten times more than the raw rubber.
2.The elasticity of the raw rubber is very high.
Choose the correct option.
A.(i) is true (ii) is false.
B.(i) is false (ii) is true.
C.Both (i) and (ii) are true.
D.Both (i) and (ii) are false
Solution
Chemically, the natural rubber is a 1,4-addition polymer of isoprene. The IUPAC name of isoprene is 2-methyl-1,3-butadiene. In natural rubber, all the double bonds have cis-stereochemistry and is also known as cis-polyisoprene.
Complete step by step answer:
The structure of natural rubber is given below:
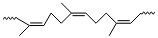
Inspection of the structure of natural rubber reveals that there is no polar group, hence the intermolecular forces of attraction are only weak van der Waals interaction. This interaction is further weakened due to cis configuration as a result the cis polyisoprene doesn't have straight chain instead has coiled structure. Also, it can be stretched like a spring and this is why natural rubber is elastic.
During the vulcanization of natural rubber, sulphur bridges or cross-linkage between polymer chains are introduced either in their allylic position or at the sites of the double bond. This cross-linkage of sulphur increases the tensile strength of the vulcanized rubber ten times of that in natural rubber. Hence both statements are correct.
So, the correct answer is Option C .
Note:
Vulcanization is defined as the process of heating the raw rubber with sulphur at 373-415 K. This process is carried out to improve the property of natural rubber. As a result of vulcanization, there is observed cross-linkage of sulphur which makes the rubber hard, non-sticky, strong, and removes the thickness of natural rubber. Since the process of vulcanization is slow therefore additives like zinc oxide are used to accelerate the rate of the process. The extent of the hardness of vulcanized rubber depends upon the amount of sulphur added.
