Question
Question: Name two metals which will displace hydrogen from dilute acids: A. \({\rm{Mg}}\)and \({\rm{Zn}}\) ...
Name two metals which will displace hydrogen from dilute acids:
A. Mgand Zn
B. Cuand Au
C. Cuand Mg
D. Znand Cu
Solution
We know that a reaction where displacement of a less reactive metal from its compound occurs by a more reactive metal is termed as displacement reaction. The reactivity of metals is decided by the reactivity series of metals.
Complete step-by step answer:
Now, we discuss the condition in which displacement of hydrogen from dilute acids takes place. From dilute acids like hydrochloric acid or sulphuric acid, hydrogen is replaced by metals which are more reactive than hydrogen. The reactivity series of metals is given as follows:
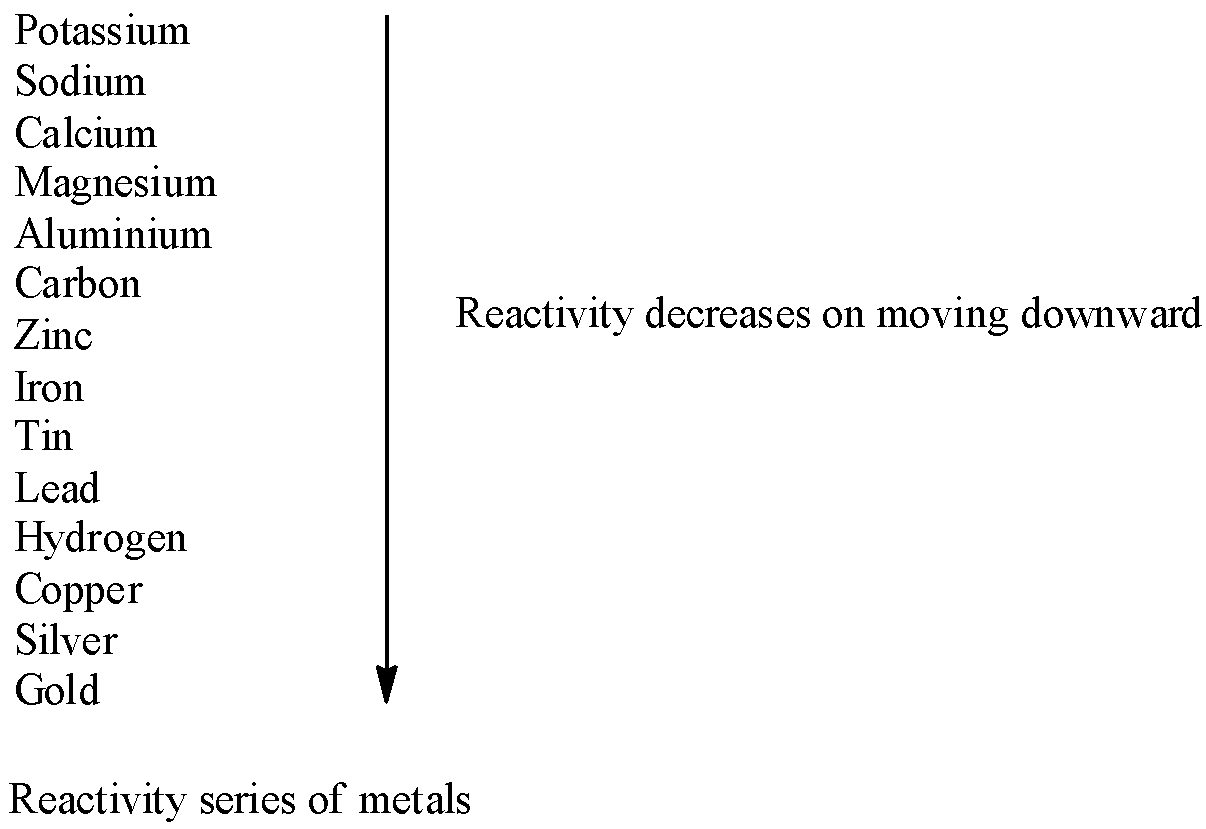
Let’s find the correct option.
Option A contains Mg(Magnesium) and Zn(zinc). Both magnesium and zinc are more reactive than hydrogen in the activity series. So, they displace hydrogen from dilute acids.
Option B contains Cu(copper) and Au(gold). Copper and gold both are less reactive than hydrogen. So, they do not displace hydrogen from dilute acids.
Option C contains Cu(copper) and Mg(magnesium). Copper is less reactive than hydrogen but magnesium is more reactive than hydrogen. So, displacement of hydrogen from dilute acid is possible in case of magnesium but not possible in case of copper.
Option D contains Zn(zinc) and Cu(copper). Copper is less reactive than hydrogen but zinc is more reactive than hydrogen. So, displacement of hydrogen from dilute acid is possible in case of zinc but not possible in case of copper.
Hence, correct option is A, that is, Mgand Zn
Note: Highly reactive metal like potassium, sodium and calcium displaces hydrogen from dilute acids with explosive violence, moderately reactive metals like magnesium, aluminium and zinc displaces hydrogen vigorously and metals such as copper, gold, mercury and silver do not displaces hydrogen from dilute acids.
