Question
Question: Name two \[\alpha -\operatorname{amino acids}\], which form a dipeptide, that is hundred times sweet...
Name two α−aminoacids, which form a dipeptide, that is hundred times sweeter than cane sugar? (This question has multiple correct options)
a) Aspartic acid
b) Alanine
c) Serine
d) Phenylalanine
e) Tryptophan
Solution
The artificial sweetener in question is Aspartame, which is hundred times sweeter than cane sugar. It is a methyl ester of dipeptide formed between two α−aminoacids.
Complete step by step answer:
The dipeptide which is hundred times stronger than sucrose (table sugar) is aspartame. It is an artificial sweetener which was accidentally discovered by a chemist named James Schlatter in the year 1965. It is presently the most used substance to give a sweet taste to various food items such as sweets, yogurts, beverages and confectionery.
Aspartame is a high calorific compound because it is completely digested in our digestive tracts after ingestion into methanol, phenylalanine and aspartic acid. But as it is many times sweeter than sucrose, even very small amounts can do the magic. In acute quantities it does not pose any threat to our bodies.
So now we know about its composition. Both phenylalanine and aspartic acids are naturally occurring amino acids and we can easily synthesize them artificially. As it is meant to be produced in the factories, the methods are a little different from the laboratory approach as they are aimed at mass production. We can go slowly over them as follows:
- phenylalanine is first reacted with methanol (an alcohol) which produces an ester
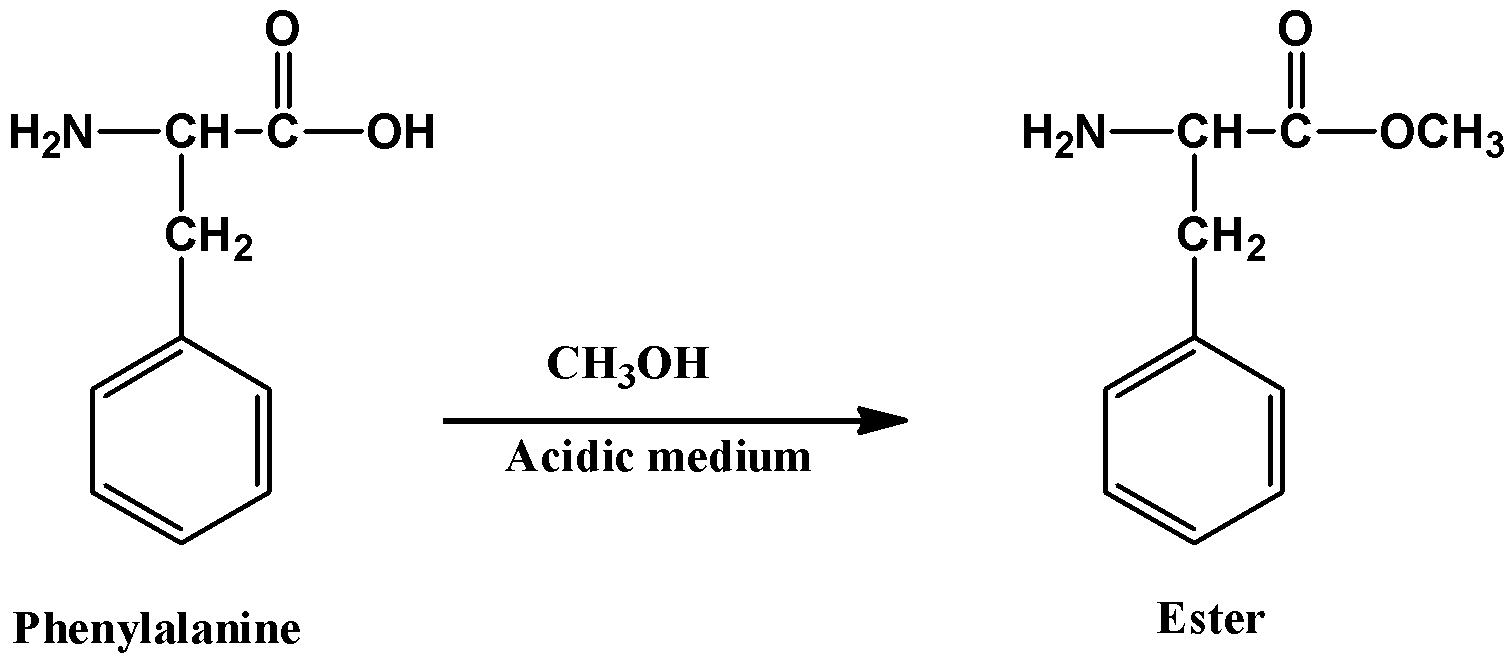
- the ester that formed above reacts with the other amino acid which is aspartic acid
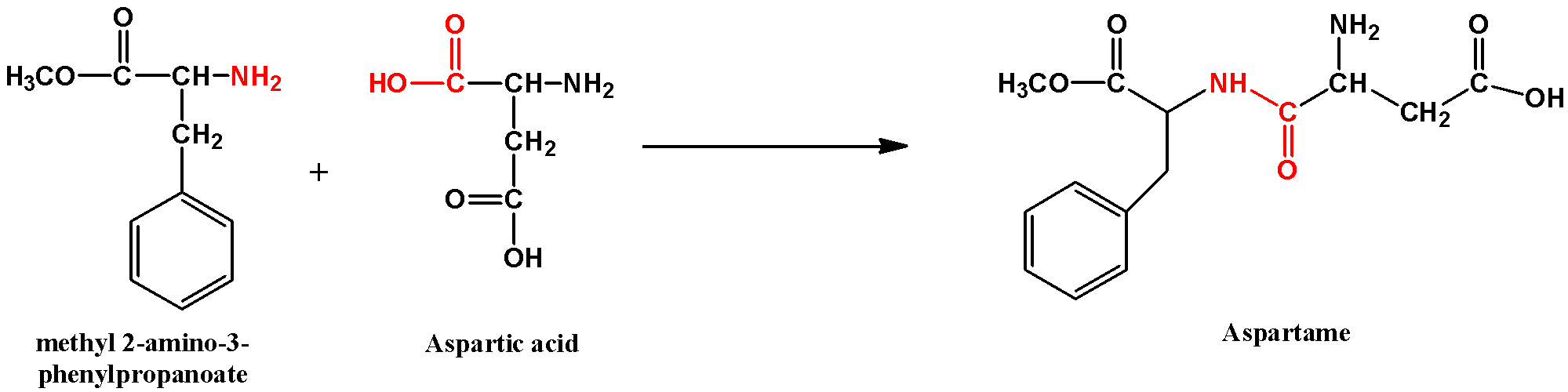
The part marked in red in the aspartame molecule is the peptide bond. This bond is an amide linkage that only forms when an acid reacts with an amine group. The reaction involves loss of a water molecule; hence it is a dehydration reaction. The peptide bond is planar in nature and is relatively stronger than normal double bonds because of resonance.
So, the correct answer is “Option A and D”.
Note: Like many other peptides, aspartame may hydrolyse (break down) into its constituent amino acids under conditions of elevated temperature or high pH. This makes aspartame undesirable as a baking sweetener, and prone to degradation in products hosting a high pH, as required for a long shelf life.
