Question
Question: Name the simplest hydrocarbon....
Name the simplest hydrocarbon.
Solution
Hint: Simplest hydrocarbon belongs to the saturated class of hydrocarbons having a single bond in their structure.
Complete answer:
In organic chemistry a hydrocarbon is a chemical compound which consists of carbon atoms and hydrogen atoms. These hydrocarbons occur naturally and they are the basis of natural gas, coal, crude oil and other energy resources.
Types of hydrocarbons:
Hydrocarbons are classified as saturated hydrocarbons, unsaturated hydrocarbon and aromatic hydrocarbons.
Saturated hydrocarbons are the simplest form of hydrocarbons they consists of single bond and are saturated with hydrogens, the formula for acyclic hydrocarbon(open chain compound) i.e alkanes is CnH2n+2 where n is the number of carbon atom.
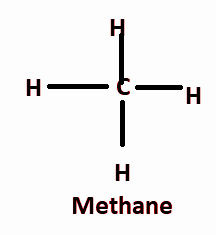
And methane is the simplest hydrocarbon which contains one carbon bonded to four hydrogen atoms.
Unsaturated hydrocarbons consists of one or more double bonds and triple bonds between the carbon atoms, those having double bonds they are called as alkenes and they have the formula CnH2n and those having triple bonds they are called as alkynes and they have the formula CnH2n−2.
Third one are aromatic hydrocarbons, they are also known as arenes(having a sigma bond and delocalized pi electrons between carbon atoms forming a circle) and they have at least one aromatic ring or we can say cyclic ring in their structure.
Note: Methane is the simplest alkane and alkanes have the formula CnH2n+2 where n is the number of carbon atom, so methane contains one carbon atom and the number of hydrogen atoms are four i.e. C1H2×1+2=CH4.
