Question
Question: Name the reagents used to convert phenol into 1.picric acid 2.2,4,6-Tribromophenol 3.Benzene ...
Name the reagents used to convert phenol into
1.picric acid
2.2,4,6-Tribromophenol
3.Benzene
4.o-phenolsulfonic acid
Solution
Before approaching the reaction, we should know the formulae, of what the reactant is, what the product is needed in the question. Only then our approach to the question will work.
Complete step by step answer:
1.Converting Phenol into picric acid.
Formula of Phenol: C6H5OH
Formula of picric acid: C6H3N3O7
Reagent required: concentrated nitric acid(HNO3), concentrated sulphuric acid (H2SO4)
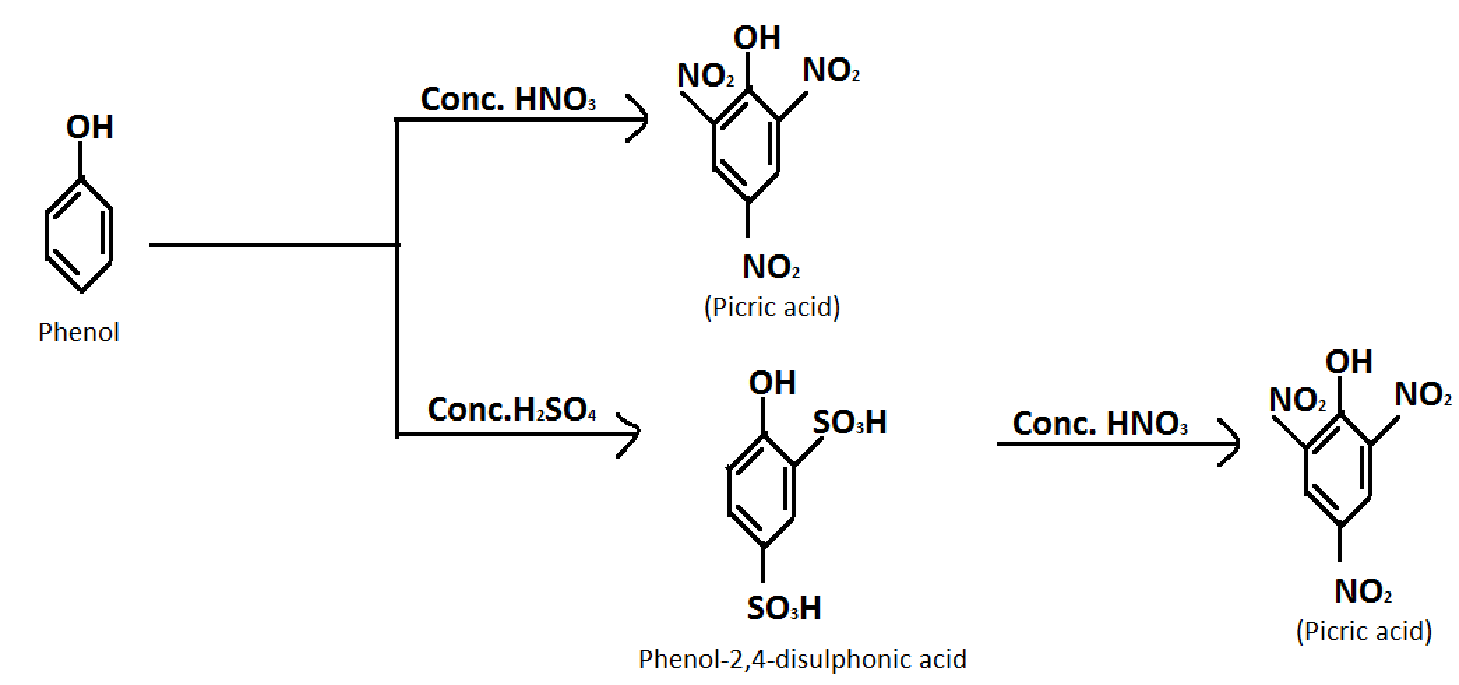
In the first reaction, the yield is poor. So we prefer the second reaction for good yield.
2.Converting phenol into 2,4,6-Tribromophenol
Formula of 2,4,6-Tribromophenol:C6H3Br3O
Reagent required: Bromine water
Reaction occurs as follows:
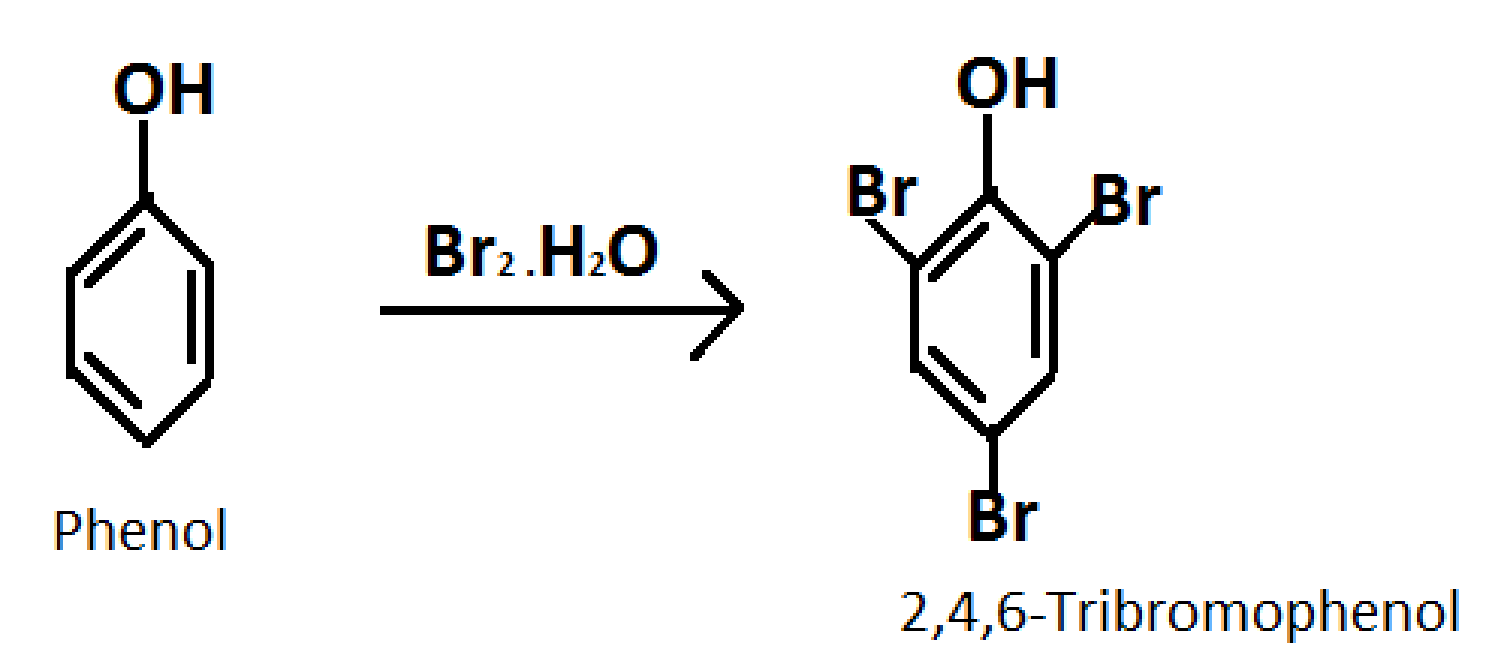
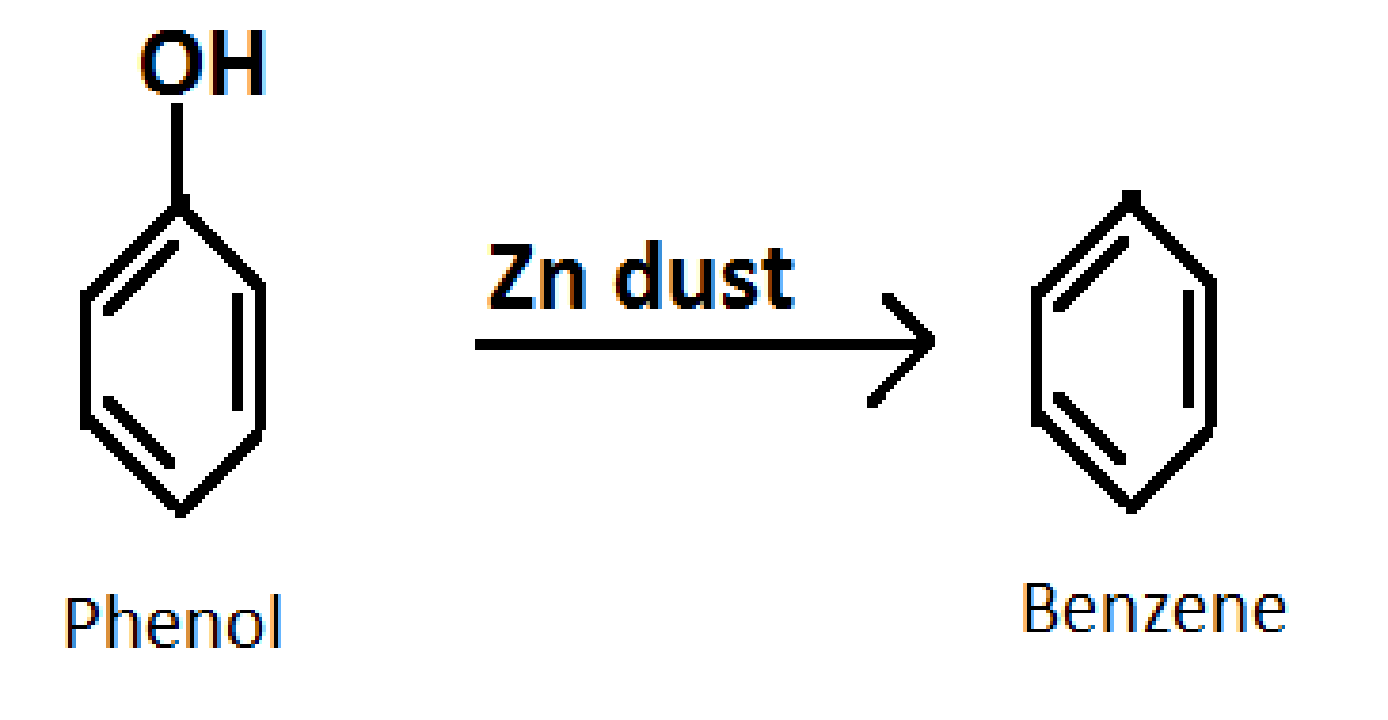
3.Converting phenol into benzene
Formula of Benzene: C6H6
Reagent required: Zinc (Zn) dust
Reaction:
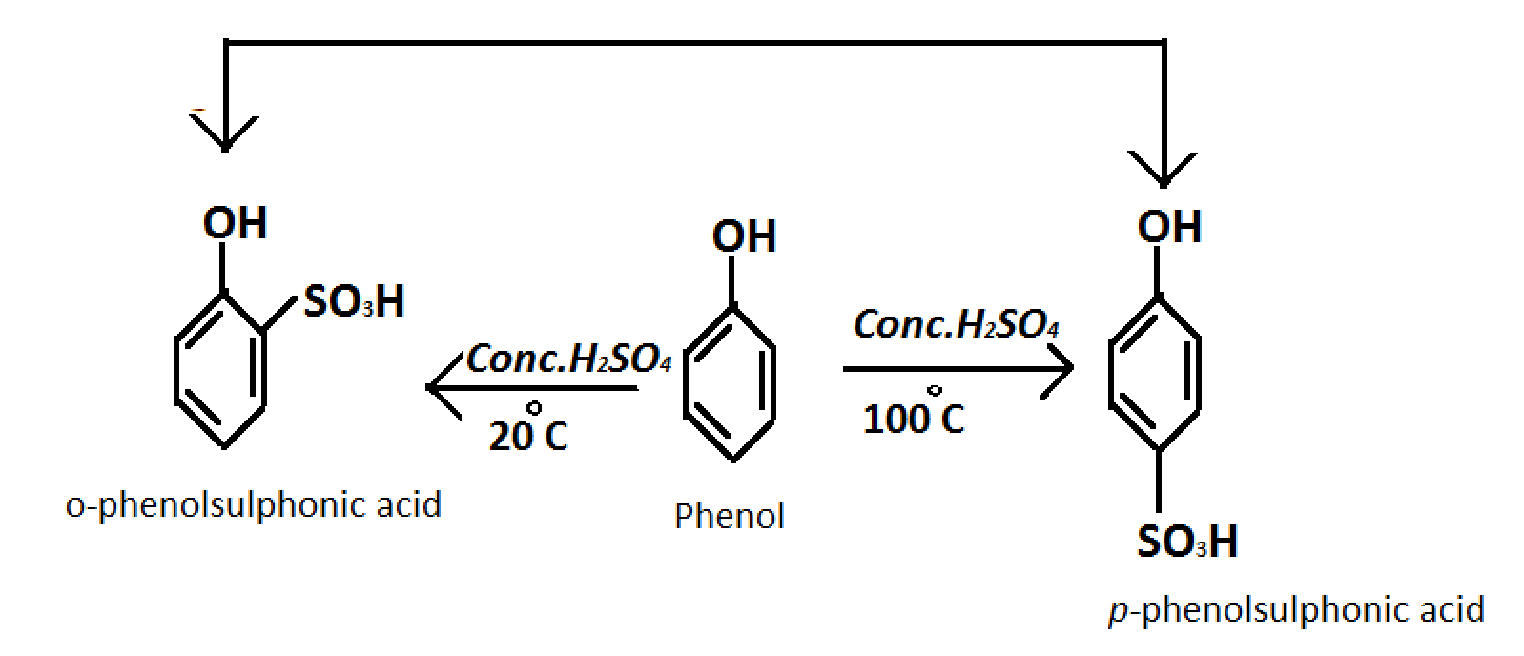
Converting phenol into a-phenolsulfonic acid Formula of Benzene: C6H6O4S
Reagent required: concentrated sulphuric acid (H2SO4) at 200C
Reaction:
Note:
These are the most important reactions from the exam point of view. The reaction of Converting phenol into 2,4,6-Tribromophenol using bromine water is a lab test for checking the −OHfunctional group. Phenol decolorizes the bromine water and gives us the white precipitates of 2,4,6-Tribromophenol.
