Question
Question: Name the method you shall use to separate two immiscible liquids....
Name the method you shall use to separate two immiscible liquids.
Solution
“Immiscible liquids are those which won't mix to give a single phase. Oil and water are examples of immiscible liquids. One floats on top of the other”.
Complete step by step answer:
We know that the property of immiscibility is going to depend on the polarity of the liquids.
-If two liquids are polar in nature they will miscible in one another very easily.
-If two liquids are non-polar then also they will miscible very easily.
-If one liquid is polar and the other liquid is non-polar they won’t miscible very easily and then they are called immiscible liquids.
-But it is not that much easy to separate the two immiscible liquids.
-There is a separate technique to separate those immiscible liquids.
The name of the instrument which is used to separate two immiscible liquids is called as separating funnel.
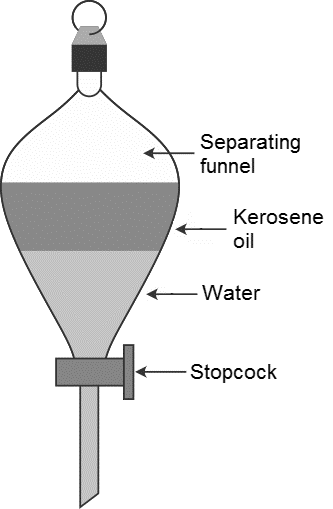
-By using the separating funnel we can easily separate two immiscible liquids.
-We have to take the two immiscible liquids into the separating funnel and close the separating funnel with a knob and leave it for a few minutes till the two layers separated.
-In the picture itself it shows that the kerosene and water are two immiscible liquids separated.
-The kerosene floats on the surface of water.
-After separation of two immiscible layers remove the knob and collect the layers separately by using a stopcock..
-First by using the stopcock we can collect the water and then kerosene in two separate beakers.
Note:
Don’t be confused with the words polar and non-polar.
Polar means the chemicals which are soluble in water are called polar.
Non-polar means the chemicals which are not soluble in water are called as non-polar.
