Question
Question: Name the following: Name the acids present in the following orange, tamarind, apples, sour milk....
Name the following:
Name the acids present in the following orange, tamarind, apples, sour milk.
Solution
Acid is a species that can either donate a proton, or accept an electron pair from suitable base. Acidic aqueous solutions have more number of hydronium ions than hydroxide ions.
Complete answer:
For a neutral solution, the pH of the solution is equal to 7. For an acidic aqueous solution, the pH is less than 7. For a basic solution, the pH is more than 7. pH is a negative logarithm of hydronium ion concentration. Hydronium ion is obtained when a water molecule is protonated. Acid donates a proton. Base accepts a proton. The reaction between acid and base is neutralization reaction. The products are salt and water.
Some examples of acids include nitric acid, oxalic acid, sulphuric acid, hydrochloric acid and acetic acid respectively.
Acids can be classified into strong acids and weak acids. Strong acids completely dissociate in aqueous solutions whereas weak acids dissociate to a small extent.
Orange contains ascorbic acid. Ascorbic acid is also called vitamin C. Tamarind contains tartaric acid. Apples contain malic acid. Sour milk contains lactic acid.
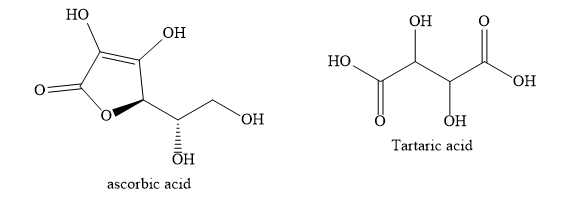
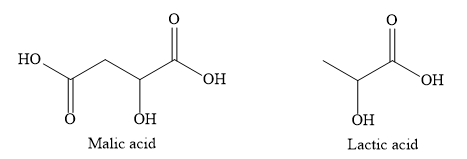
Note: Acids can be classified as monobasic, dibasic, tribasic etc, depending on the number of hydrogen ions that can be obtained from one molecule of the acid. Monobasic, dibasic, tribasic acids give one, two and three protons respectively per molecule of the acid.
