Question
Question: Name the following coordination entities and describe their structures: i. \({\left[ {{\text{Fe}}{...
Name the following coordination entities and describe their structures:
i. [Fe(CN)6]4−
ii. [Cr(NH3)4Cl2]+
iii [Ni(CN)4]2−
(Atomic numbers Fe=26, Cr=74, Ni=28)
Solution
The strong field ligands cause the pairing of the electrons in the d-orbitals and thus forming low spin complexes. Whereas the weak field ligands do not cause the pairing of electrons and form high spin complexes.
Complete step by step solution:
i. [Fe(CN)6]4−:
- Write the IUPAC name of [Fe(CN)6]4− as follows:
Determine the oxidation number (ON) of the central metal atom Fe as follows:
ON of Fe + 6×ON of CN−=Charge of the complex ion
Substitute (−1) for the oxidation number of CN−, −4 for the charge on the complex. Thus,
ON of Fe + 6×(−1)=−4
ON of Fe=−4+6
ON of Fe=+2
Thus, the oxidation number (ON) of the central metal atom Fe is +2.
Write the IUPAC name of [Fe(CN)6]4− as follows:
The charge on the complex ion is negative. Thus, [Fe(CN)6]4− is a complex anion.
Write the name of the ligands. There are six cyanide ligands. Thus, hexacyano.
Write the name of the metal ending with suffix –ate. Thus, ferrate.
Write the charge on the iron atom in the parenthesis. The charge on the Fe atom is +2.
Thus, the IUPAC name of [Fe(CN)6]4− is hexacyanoferrate (II).
- Determine the structure of [Fe(CN)6]4− as follows:
The oxidation state of the Fe metal is +2. Thus, the electronic configuration of Fe2+is 3d64s04p0.
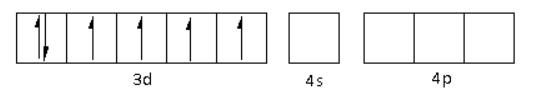
The CN− is a strong field ligand. Thus, it causes the pairing of electrons in the 3d orbital. Thus,
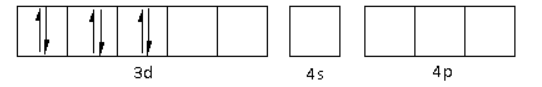
There are six CN− ligands around the Fe metal atom. The six electron pairs from the six CN− ligands occupy the d2sp3 orbitals as follows:
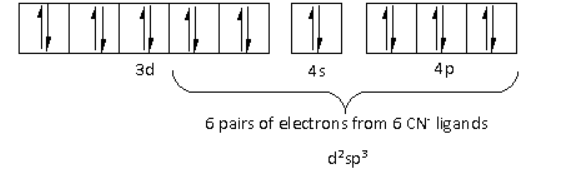
Thus, the hybridisation of [Fe(CN)6]4− is d2sp3 and the structure is octahedral.
ii. [Cr(NH3)4Cl2]+
- Write the IUPAC name of [Cr(NH3)4Cl2]+ as follows:
Determine the oxidation number (ON) of the central metal atom Cr as follows:
ON of Cr + 4×ON of NH3 + 2×ON of Cl−=Charge of the complex ion
Substitute (−1) for the oxidation number of CN−, 0 for the oxidation number of NH3, −1 for the oxidation number of Cl−, +1 for the charge on the complex. Thus,
ON of Cr + 4×(0) + 2×(−1)= + 1
ON of Cr=+1+2
ON of Cr=+3
Thus, the oxidation number (ON) of the central metal atom Cr is +3.
Write the IUPAC name of [Cr(NH3)4Cl2]+ as follows:
The charge on the complex ion is positive. Thus, [Cr(NH3)4Cl2]+ is a complex cation.
Write the name of the ligands. There are four amine ligands and two chlorine ligands. Thus, tetraammine dichloride.
Write the name of the metal. Thus, chromium.
Write the charge on the chromium atom in the parenthesis. The charge on the Cr atom is +3.
Write the word ion at the end.
Thus, the IUPAC name of [Cr(NH3)4Cl2]+ is tetraammine dichloride chromium (III) ion.
- Determine the structure of [Cr(NH3)4Cl2]+ as follows:
The oxidation state of the Cr metal is +3. Thus, the electronic configuration of Cr3+is 3d34s04p0.
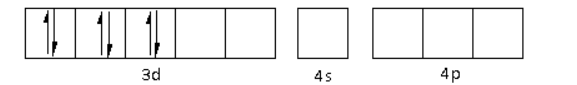
There are a total six ligands around the Cr metal atom. The six electron pairs from the six ligands occupy the d2sp3 orbitals as follows:
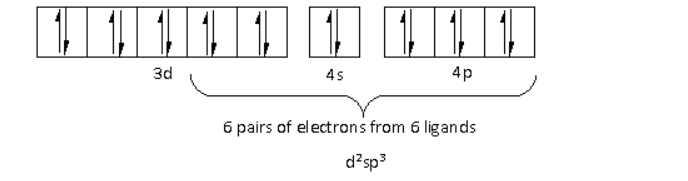
Thus, the hybridisation of [Cr(NH3)4Cl2]+ is d2sp3 and the structure is octahedral.
iii. [Ni(CN)4]2−:
- Write the IUPAC name of [Ni(CN)4]2− as follows:
Determine the oxidation number (ON) of the central metal atom Ni as follows:
ON of Ni + 4×ON of CN−=Charge of the complex ion
Substitute (−1) for the oxidation number of CN−, −2 for the charge on the complex. Thus,
ON of Ni + 4×(−1)=−2
ON of Ni=−2+4
ON of Ni=+2
Thus, the oxidation number (ON) of the central metal atom Ni is +2.
Write the IUPAC name of [Ni(CN)4]2− as follows:
The charge on the complex ion is negative. Thus, [Ni(CN)4]2− is a complex anion.
Write the name of the ligands. There are four cyanide ligands. Thus, tetracyano.
Write the name of the metal ending with suffix –ate. Thus, nickelate.
Write the charge on the nickel atom in a bracket. The charge on the nickel atom is +2.
Thus, the IUPAC name of [Ni(CN)4]2− is tetracyanonickelate (II).
- Determine the structure of [Ni(CN)4]2− as follows:
The oxidation state of the Ni metal is +2. Thus, the electronic configuration of Ni2+is 3d84s04p0.
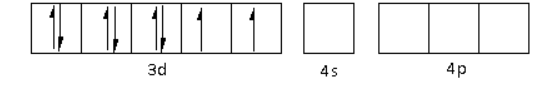
The CN− is a strong field ligand. Thus, it causes the pairing of electrons in the 3d orbital. Thus,
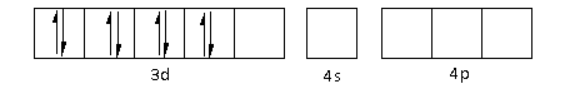
There are four CN− ligands around the Ni metal atom. The four electron pairs from the six CN− ligands occupy the dsp2 orbitals as follows:
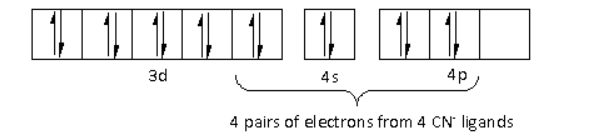
Thus, the hybridisation of [Ni(CN)4]2− is dsp2 and the structure is square planar.
Note:
The rules for writing the IUPAC nomenclature of complex anions are as follows:
1. Identify the ligand and name the ligand with the prefix mono-, di-, tri-, tetra-, etc. depending on the number of ligands.
2. Identify the central metal atom and write the name of the central metal atom ending with suffix –ate.
3. Determine the oxidation number of the central metal atom and write the charge in Roman numeral in a parenthesis after writing the complete name.
The rules for writing the IUPAC nomenclature of complex cations are as follows:
1. Identify the ligand and name the ligand with the prefix mono-, di-, tri-, tetra-, etc. depending on the number of ligands.
2. Identify the central metal atom and write the name of the central metal atom.
3. Determine the oxidation number of the central metal atom and write the charge in Roman numeral in a parenthesis after writing the complete name.
4. Write the word ‘ion’ after writing the charge.
