Question
Question: Name the following compounds. 1\. 
2. 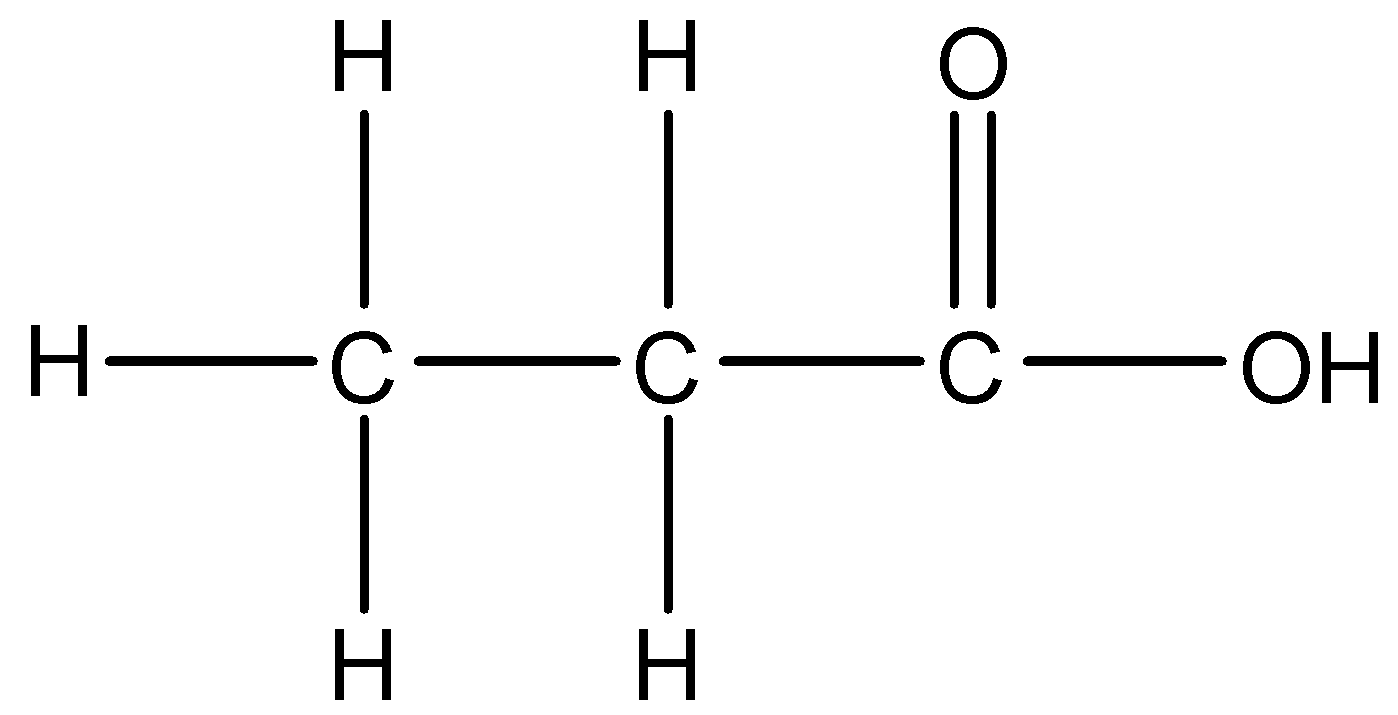
3. 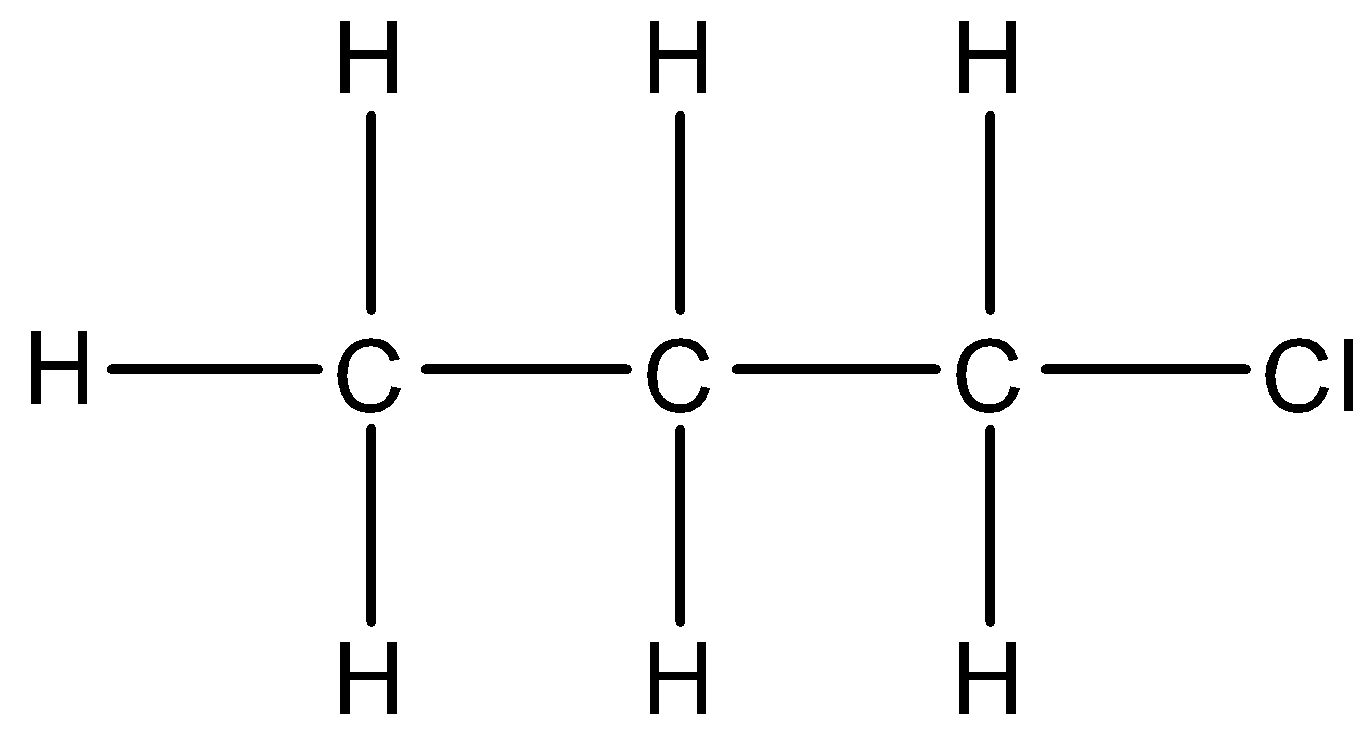
4.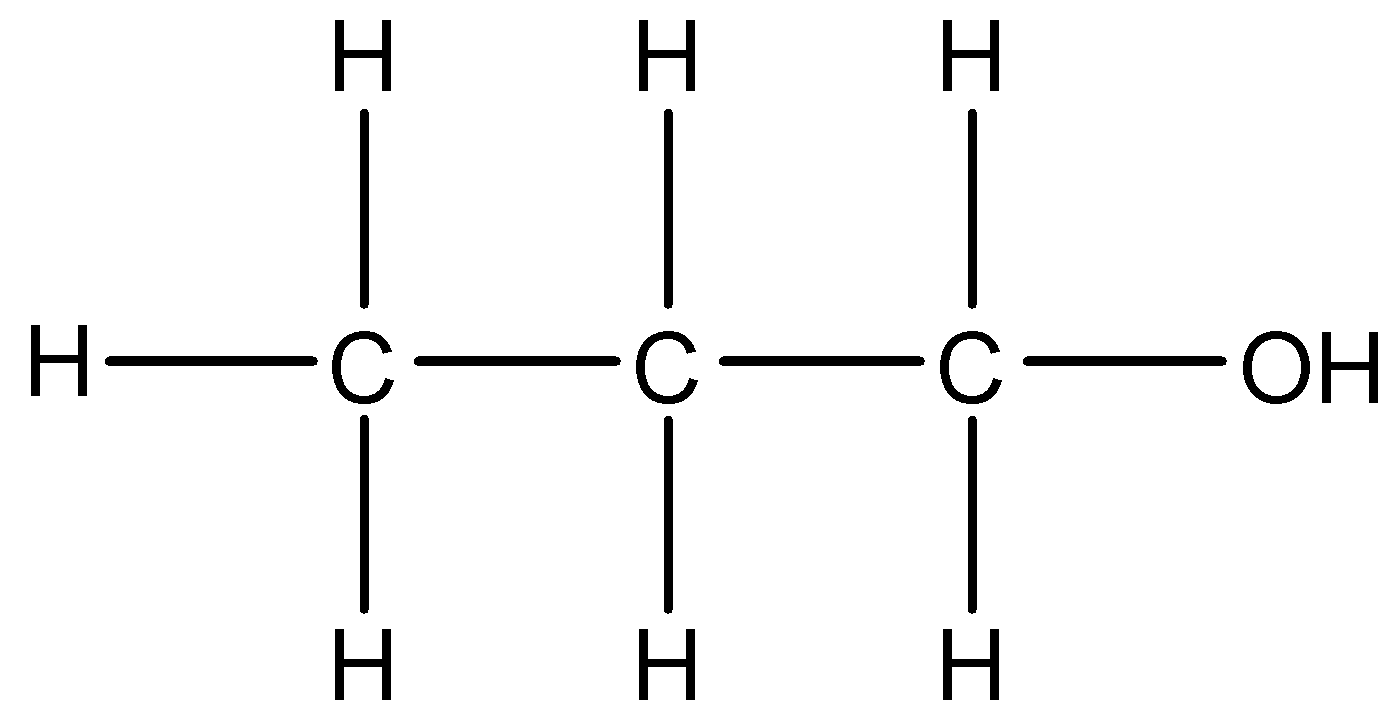
5. 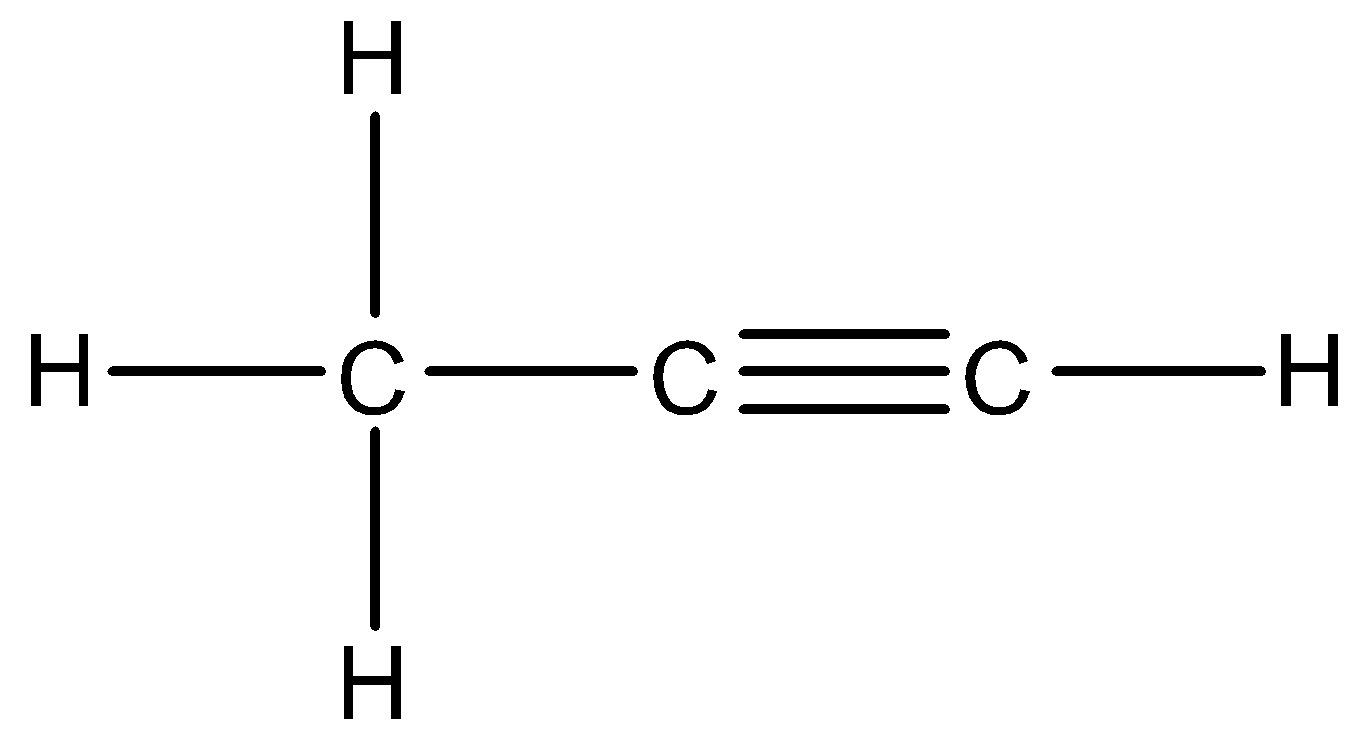
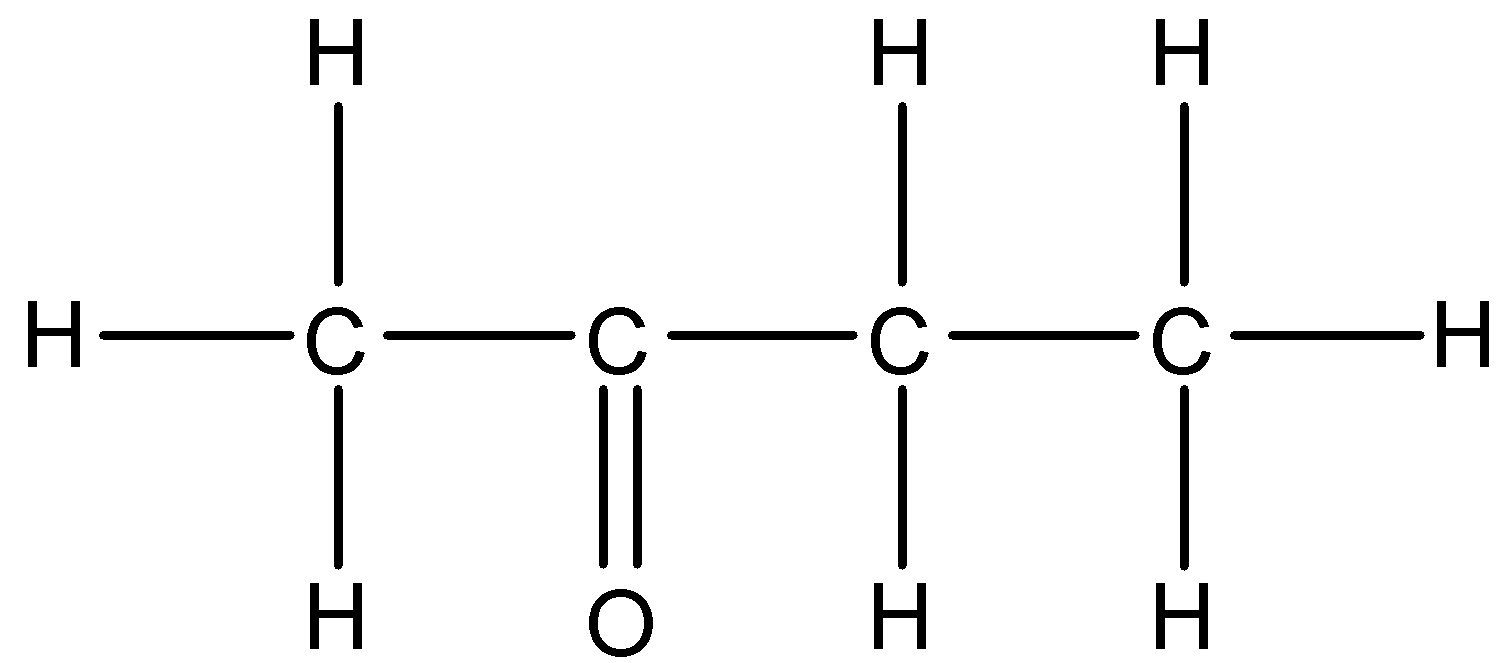
6. 
Solution
To name a compound, first find the functional group present in the compound. Then find the longest chain of carbon atoms present in the compound in a way that the carbon of the functional group is involved in the chain.
Complete answer:
We will give the name according to IUPAC nomenclature to all the given compounds one by one.
- To name a compound, first find the functional group present in the compound. Then find the longest chain of carbon atoms present in the compound in a way that the carbon of the functional group is involved in the chain. Then use suitable suffixes of the functional group and indicate the positions of substituent groups as well.
1)
We can see that –OH group is present in the compound. The longest chain of carbon atoms present in the compound is of three carbons. We will use the suffix –ol. Thus, the IUPAC name of this compound will be Propan-1-ol.
2)
There is a –COOH group present in the compound. We know that –oic acid is the suffix used to describe the presence of acids. The parent carbon chain is of three carbons. We will start numbering the chain in a way that the carbon of the functional group gets the lowest number. Thus, the IUPAC name of this compound is 1-Propanoic acid.
3)
There is a chlorine atom in the structure. The parent carbon chain is of three carbons. We will take chlorine atoms as a substituent and use chloro- prefix. So, the IUPAC name of this compound will be 1-chloropropane.
4)
This compound is the same as the first compound.
5)
There is a carbon carbon triple bond present in the compound. Thus, this is an alkyne and –yne suffix is used to indicate this functional group. The parent carbon chain consists of three carbons. So, IUPAC name of this compound will be 1-Propyne.
6)
We can see that this compound contains a ketone functional group. So, we will use –one suffix. The parent carbon chain contains four carbon atoms. So, the IUPAC name of this compound will be Butan-2-one.
Note:
When more than one type of functional group is present in the compound, then we need to write them in alphabetical order. If one substituent is present in the compound more than one time, then we can use prefixes like di-, tri-, tetra- etc.
