Question
Question: Name the following: A metal oxide that can be reduced by hydrogen....
Name the following:
A metal oxide that can be reduced by hydrogen.
Solution
We know that metal oxide is the compound which is composed of a metal cation and oxide anion. They generally exist in solid crystalline forms. Some examples of metal oxide are iron oxide, zinc oxide, copper oxide etc.
Complete step by step answer: First, we discuss the activity series of metals. This series of metals is the listing of metals according to their reactivity. Metals of high reactivity are listed at the top of the series and the less reactive metals are listed at the bottom.
We know that a reaction in which displacement of a less reactive metal from its compound takes place by a more reactive metal is termed a displacement reaction. The activity series is given below.
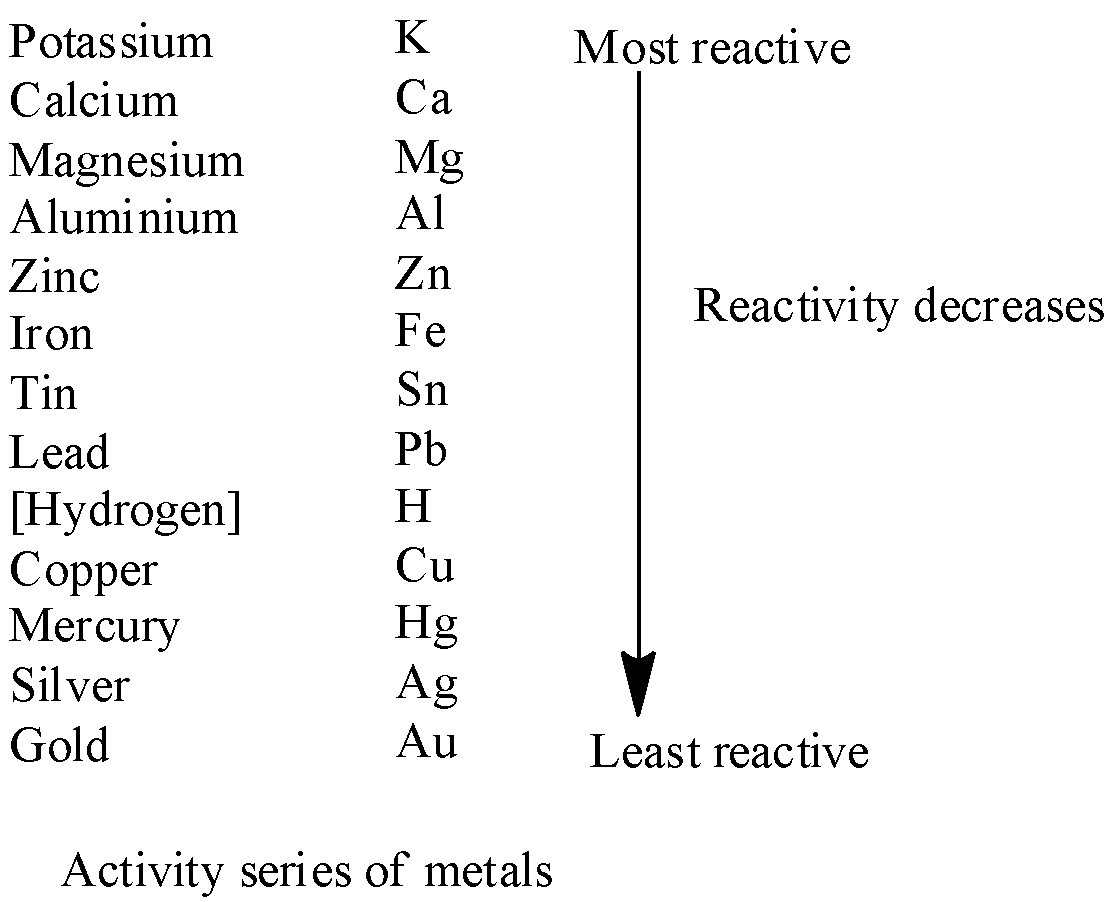
Considering the above series we can say that hydrogen can reduce only oxides of copper, mercury, silver and gold as these metals are less reactive than hydrogen. The reduction of oxides of tin, iron, zinc, magnesium, calcium, potassium is not possible by hydrogen as these metals are more reactive than hydrogen.
Let’s take the example of copper oxide. From the activity series, we can clearly say that it is less reactive than oxygen so hydrogen will reduce it.
Now, we write the reduction reaction of copper oxide by hydrogen.
CuO+H2→Cu+H2O
The reaction produces copper and water.
Hence, one metal oxide that is reduced by hydrogen is copper oxide (CuO)
Note: Remember that a chemical reaction in which oxygen is removed or hydrogen is added or electrons are gained, then this type of reaction is termed as reduction reaction. In the reaction of copper oxide and hydrogen, oxygen is removed from copper oxide, so it is a reduction reaction.
