Question
Question: Name the compound with bond angles that are exactly \( 109 \) and degree \( 28 \) ....
Name the compound with bond angles that are exactly 109 and degree 28 .
Solution
To answer this question we should have a basic idea about Bond angles. Bond angle can be defined as the angle made by two covalent bonds, these two covalent bonds are produced by the same atom. We will discuss more about the bond angle in this question to understand it better for identifying the required compound.
Complete answer:
Bond angle is a Bond property or bond parameter that provides information about the molecular geometry of the compound. Bond angle depends upon several factors which are
Number of lone pairs: If there are more lone pairs in the compound then there will be lone pair-bond pair repulsion and bond angle is reduced.
Size of central atom and surrounding atoms: If the size of atoms is larger than the electrons have more space to occupy and they face less repulsion from other electrons and gap between bond pairs increases resulting in increasing the bond angle.
Electronegativity of central atoms of surrounding atoms: If atoms are more electronegative than the electrons will be attracted more towards them resulting in decreasing the bond angle.
A compound named Carbon tetrachloride has bond angles that are exactly 109 and 28 degrees. let’s see its structure
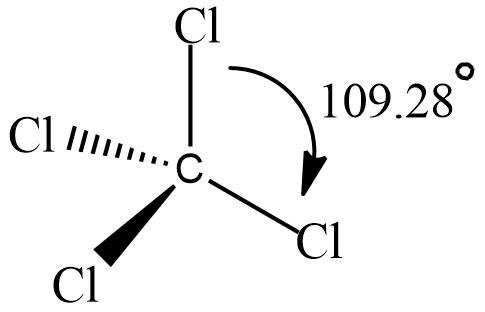
It has tetrahedral geometry and each C−Cl bond has a bond angle 109.28∘.
Note:
The bond angle provides us a rough idea on location of lone pairs as well as bond pairs, it also tells us about the location of the central atom and surrounding atoms in the compound so that we can determine the shape and geometry of the compound.
