Question
Question: Name the chemical used to test starch....
Name the chemical used to test starch.
Solution
The chemical belongs to the group of halogens and is a non-metallic solid with a purple-black luster in the standard conditions but it melts to form a deep violet liquid when the temperature is around 144 degrees celsius.
Complete answer:
Iodine is used to test the presence of starch and this test is known as Starch-Iodine test. In this test, the presence of starch is detected by observing a change in color that is due to the formation of a complex. The reagents in this test are – a solution of I2 (iodine) and KI (potassium iodide). The solution of these two chemicals in water is generally light orange-brown but when it is added to another solution or sample that contains starch, the light orange-brown color changes to deep blue. This change in color is due to the charge-transfer complexes. In the presence of potassium iodide, the iodine forms polyiodide ions like I3− that act as charge donors and get embedded in the amylose helix forming a complex that is deep blue.
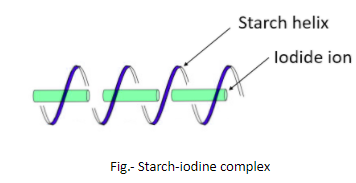
So, it is clear now that it is the amylose in the starch which is responsible for the formation of deep blue color by forming a complex with iodine. Iodine is a lustrous purple-black non-metallic solid belonging to the group of halogens.
Note: There is a limitation of this test that it cannot be performed in a very low pH environment because in such conditions, the hydrolysis of starch occurs and it prevents the formation of a starch-iodine complex that gives the blue color as an indication of the presence of starch.
