Question
Question: Name the acids present in vinegar, grapes, and lemon....
Name the acids present in vinegar, grapes, and lemon.
Solution
Hint: The acid present in vinegar is an organic aliphatic carboxylic acid with two carbon atoms. In dilute solution, it is a very weak acid.
The acid present in grapes is an aliphatic acid having four carbons with the two carboxyl groups at the terminal ends and the two hydroxyl groups at the alpha positions of the two carboxyl groups.
The acid present in lemon is a weak organic acid with three carboxyl groups and one hydroxyl group.
Complete step by step answer:
1.The acid present in vinegar is acetic acid or ethanoic acid diluted in water. The chemical formula of the acid is CH3COOH. It has a characteristic sour taste and smell in the food. In water, it dissociates as CH3COO−andH+ions.
2.The acid present in grapes is tartaric acid. Other acids present in grapes are malic and citric acids. Tartaric and malic acid account for almost 70 to 90% of the grapes’ acidity.
Tartaric acid is also present in grapes and bananas. Its chemical formula is C4H6O6. The structure of the tartaric acid is:
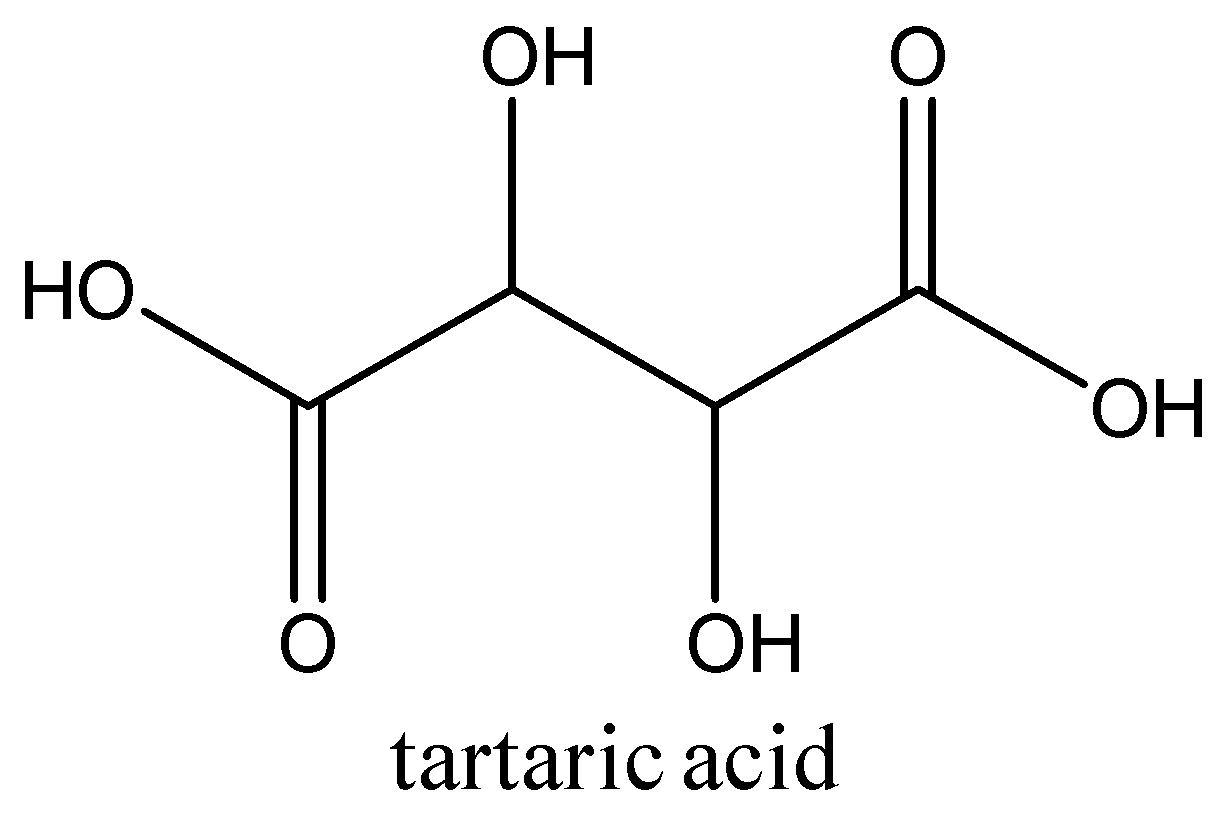
This is used mainly in the wine-making process.
3.The acid present in lemon is citric acid. Its chemical formula is C6H8O7.
The structure of the citric acid is:
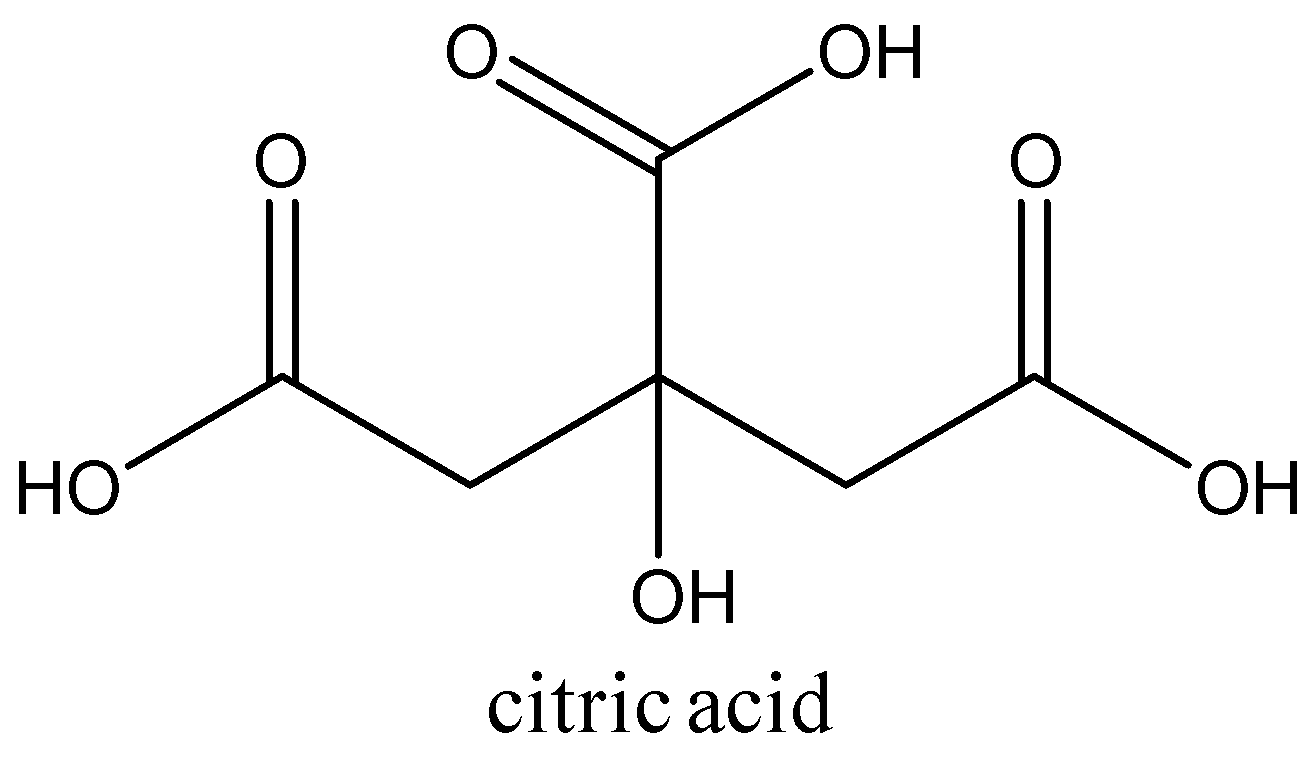
So, the acids present in vinegar, grapes and lemon are acetic acid, tartaric acid and citric acid respectively.
Note: All the three acids are toxic in nature. Acetic acid if not used safely and properly, it may corrosion to the skin and eyes. Tartaric acid when introduced in the muscle, it acts as a toxin by inhibiting malic acid and causing paralysis and death. Over ingestion of citric acid may cause breathing problems, abdominal pain and so on.
