Question
Question: Name a reagent which can distinguish between ethene and ethane....
Name a reagent which can distinguish between ethene and ethane.
Solution
The reagent reaction is a type of additional reaction.
Type of hydrocarbon does not undergo addition reactions.
Bromine water is a coloured solution which gets decolourized on reaction with the particular type of hydrocarbons.
Complete step by step answer:
Bromine test:
Bromine water used as a reagent.
Bromine water is known as bromide, bromine solution with the chemical formula Br2. Bromine water is prepared by dissolving diatomic bromine and has high oxidizing properties and is obtained as a yellow mixture. (Br2) in water (H2O).
This test is a type of addition reaction. The only non-metal which is in the liquid state is bromine. Bromine is said to have very good oxidizing property.
Bromine water can also be prepared in a chemical laboratory by mixing fumes of bromine with water.
The bromine water test is qualitative, used to identify the alkane or alkene functional groups present in the compound.
Alkane does not react with the bromine water solution and the dark yellow colour of bromine solution remains the same. Example: Ethane
H3C−CH3→Noreaction
Alkene undergoes an addition reaction. For example, ethene reacts with bromine water to give 1,2-dibromoethane.
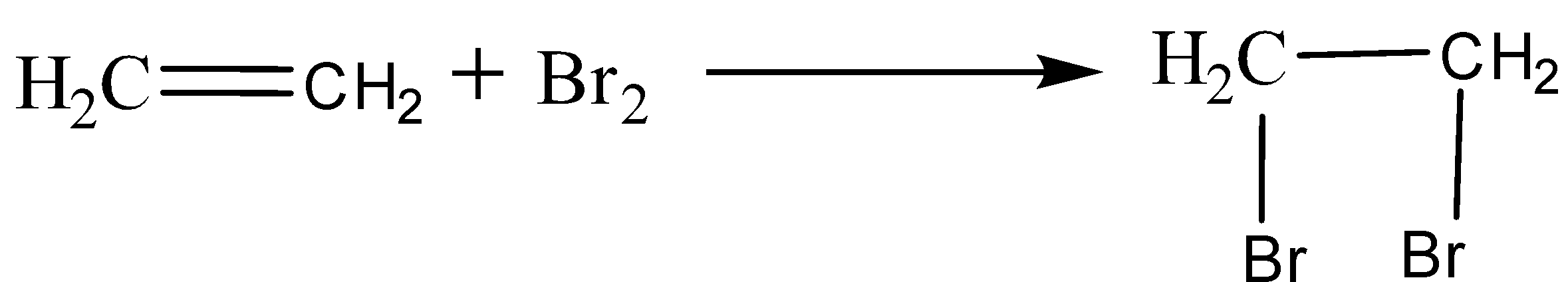
Alternative solution:
KMnO4 solution:
Ethane does not react with alkaline KMnO4, the purple colour of KMnO4 and remains the same.
Ethene reacts with alkaline KMnO4, it oxidizes ethene to give ethane 1,2-diol (ethylene glycol).
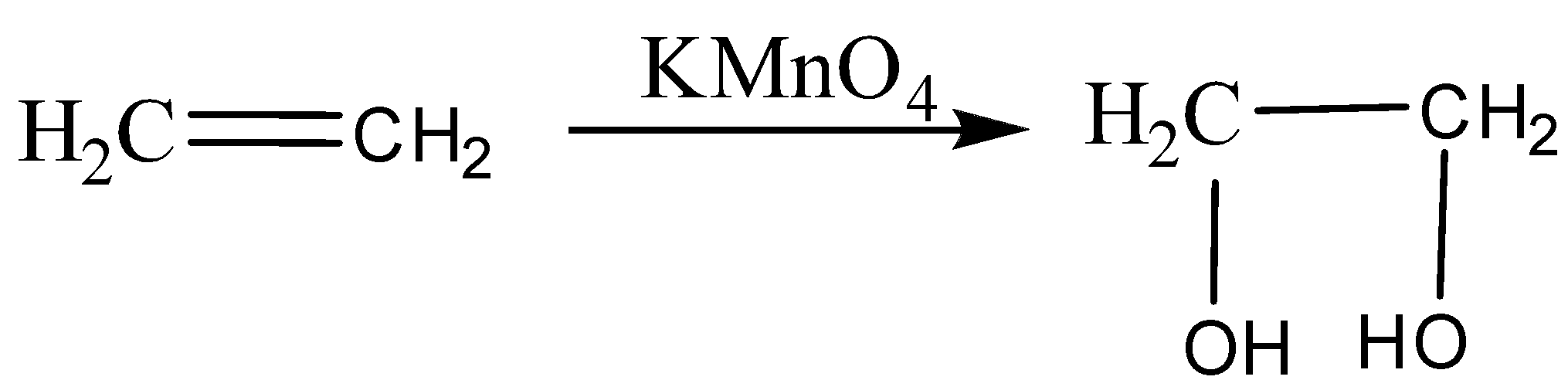
The purple colour of KMnO4 changes into colourless after oxidation.
Note:
Bromine water test works by halogenation mechanism.
Bromine water test takes place at room temperature in case of gaseous alkene but in case of liquid alkenes, the reaction will take place in presence of CCl4.
