Question
Question: Name a non-metal which is liquid at room temperature....
Name a non-metal which is liquid at room temperature.
Solution
It is a reddish-brown liquid that is extremely poisonous to humans. It falls in the halogen category and it is used to form a reagent that is used in Markovnikov's addition reaction.
Complete step-by-step solution:
In order to answer our question, we need to gain knowledge about bromine. Bromine is a nonmetal in the group 17 of the periodic table, and it is the third halogen. Bromine possesses properties that are more or less similar to other halogens like fluorine, chlorine and iodine. The electronic configuration of bromine is [Ar]3d104s24p5. It has a deficiency of one electron to fulfil its octet configuration, so for this reason, it is a good oxidising agent, however a weaker oxidising agent than chlorine. Properties of bromine are intermediate between iodine and chlorine, for example, bromine is less reactive than chlorine and more reactive than iodine. Moreover, properties like ionisation energy, electron affinity ionic radius as well as bond length of bromine fall between chlorine and iodine. Bromine is reddish to brownish in colour and the reason for it is the transition of the electrons from the highest occupied antibonding orbital to the lowest vacant antibonding π orbital. Bromine exists as a diatomic molecule Br2 and its structure looks like:
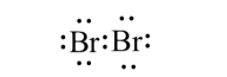
The molar mass of bromine is about 160gmol−1. There are two stable isotopes of bromine which are possible which are 79Br and 81Br. Both of them are the natural isotopes and they occur almost in equal percentage in nature. However, bromine is very toxic to humans. Liquid bromine can burn the skin of humans, whereas inhalation of the bromine gas may lead to breathing problems and adverse effects in the respiratory tract.
Hence, bromine is the non-metal which is liquid at normal room temperature.
Note: It is to be noted that the Van der waals force of attraction between the molecules increases down the group. So, chlorine is a gas, bromine a liquid and iodine a solid as their forces of attraction increase respectively.
