Question
Question: n-pentane and isopentane are _____________ isomers. \[1)\] Chain \(2)\) Position \(3)\) Geomet...
n-pentane and isopentane are _____________ isomers.
1) Chain
2) Position
3) Geometrical
4) Optical
Solution
Isomers in chemistry are molecules with identical molecular formulae, that is, the same number of atoms of each element but different atomic configurations in space. Isomerism refers to the existence or potential of isomers. Isomers don't always have the same chemical or physical properties as one another.
Complete answer: The organic compound pentane has the formula C5H12, which means it's a five-carbon alkane. The name can refer to any of three structural isomers, or a mixture of them; however, in IUPAC nomenclature, pentane only refers to the n-pentane isomer; the other two are known as isopentane (methylbutane) and neopentane, respectively (dimethylpropane).
Although chain isomers have the same molecular formula, the manner their carbon atoms are connected varies from one isomer to the next. Chain isomers exist in alkane molecules with four or more carbon atoms. The carbon atoms in these isomers are linked together in various ways to form branches.
n-pentane - It's just a five-carbon-atom straight chain.
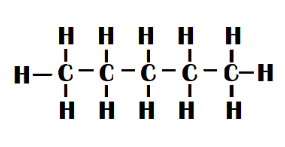
N-pentane is a white liquid with a petroleum-like odour. The flash point is 57∘F. The boiling point is 97∘F. It is less dense than water and is insoluble in it. As a result, it floats on water. Vapours weigh more than air.
Isopentane - One methyl group is linked to the second carbon of the main straight chain of four carbon atoms in this type of isomer. Methyl-butane is another name for it.
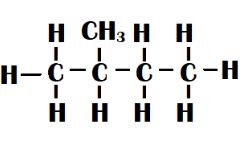
Neo-pentane - Two methyl groups are connected to the centre carbon of a straight chain of three carbon atoms in this type of isomer. Its form is tetrahedral. It is also known as dimethyl propane.
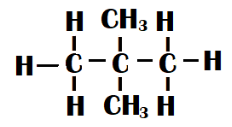
Both isomers have the same molecular formula but are different in the manner in which they are formed. Hence, they both are chain isomers.
The correct option is A.
Note:
A hierarchy of isomeric relationships exists. Two molecules with the same stereoisomer but different conformational forms or isotopologues could be in various conformational forms or be different isotopologues.
