Question
Question: n-butane and isobutane are: (A) Same compound (B) Allotropes (C) Isomers (D) Structural isom...
n-butane and isobutane are:
(A) Same compound
(B) Allotropes
(C) Isomers
(D) Structural isomers
Solution
We should know about the terms allotropes, isomerism and its types. Also, we should know how to draw the structures, the IUPAC nomenclature of these structures and the common names used for them.
Complete step by step answer:
n-butane has chemical formula C4H10 and isobutane has chemical formula C4H10 . So we observe that the chemical formulas for both are the same. Thus, allotropes option is eliminated. Now we will check the order in which they are placed.
Structural formula of n-butane can be written as CH3−CH2−CH2−CH3
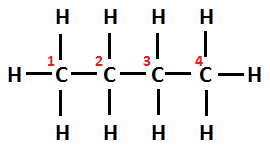
Whereas, Structural formula of isobutane can be written as CH3−CH(CH3)−CH3 . Its IUPAC name is Methylprop-2-ene.
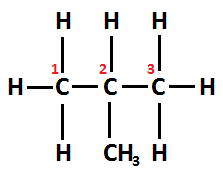
Since the chain varies due to the position of the carbon atom, thus it is an example of a structural isomer.
So, option D. structural isomer is the correct option to the given question.
Note:
| Serial number | Allotropes | Isomers |
|---|---|---|
| 1. | They have different chemical formulas. | They have the same chemical formula. |
| 2. | They are species of the same element. For example, oxygen O2 and ozone O3 . | They are composed of different elements, for example C4H8 is an example of but-1-ene and but-2-ene. |
| 3. | They always have different structures. | They may or may not have the same structures. |
| 4. | It is observed in metals, nonmetals and metalloids. | Isomerism is generally observed in inorganic molecules and hydrocarbons. |
| 5. | We can’t specify the type. For carbon, there are eight allotropes. | Types: structural isomers and stereoisomers. |
