Question
Question: \(N{{a}_{2}}{{S}_{2}}{{O}_{3}}\) is used in photography for fixing the negative. It removes AgBr by:...
Na2S2O3 is used in photography for fixing the negative. It removes AgBr by:
(A) Formation of the complex ( [Na3(AgS2O3)2] )
(B) Oxidation of Br− to Br2
(C) Reduction of Ag+ to Ag
(D) Formation of double salt
Solution
A chemical compound that has a formula Na2S2O3 is named sodium thiosulfate. The commercial name of sodium thiosulfate is called Hypo. It is a crystalline solid that tends to readily lose water and readily soluble in water is referred to as sodium thiosulfate.
Complete step by step solution:
Sodium thiosulfate structure:
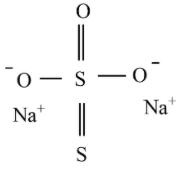
The shape of the thiosulfate ion is tetrahedral in the solid-state of sodium thiosulfate. Compared to thiosulfate ion, the distance between two sigma bonded sulfur atoms differs from the distance between the two sulfur atoms which implies that the sulfur is not bonded to any oxygen that holds a negative charge.
Physical and chemical properties of Na2S2O3 :
- Sodium thiosulfate has a white crystalline solid and is odorless.
- The density of hypo is 1.667 grams per cubic meter.
- The solubility of hypo in water is 70.1 g/100 ml at 20oC and 231g/100ml at 100oC
- The crystal structure of sodium thiosulfate crystals is monoclinic.
Na2S2O3 is used in photography for fixing the negative. It removes AgBr by Formation of the complex ( [Na3(AgS2O3)2] ) which is an undecomposed complex. The reaction of the process as follows:
AgBr+2Na2S2O3→Na3[Ag2(S2O3)2]+NaBr
Hence, the correct answer is option A.
Note: Sodium thiosulfate causes mild skin irritation, mechanical eye irritation, and causes upper respiratory tract irritation. It is used in the medical treatment of cyanide poisoning cases and used in the treatment for the side effects of chemotherapy and hemodialysis.
