Question
Question: Myoglobin stores oxygen for metabolic process in muscle. Chemical analysis shows that it contains 0....
Myoglobin stores oxygen for metabolic process in muscle. Chemical analysis shows that it contains 0.32% Fe by mass. If there is one Fe atom per molecule of myoglobin, what is the molar mass of myoglobin? [At. Mass of Fe = 56 u]
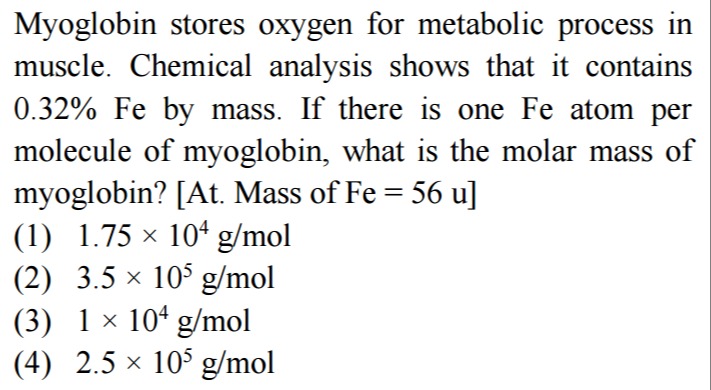
A
1.75 × 104 g/mol
B
3.5 × 105 g/mol
C
1× 104 g/mol
D
2.5 × 105 g/mol
Answer
1.75 × 104 g/mol
Explanation
Solution
Given that myoglobin contains 0.32% Fe by mass and has one Fe atom per molecule,
The mass contribution of Fe is:
Mass contribution of Fe=56 g/molLet the molar mass of myoglobin be M. Then,
0.0032×M=56⇒M=0.003256=1.75×104 g/mol