Question
Question: Mutarotation is shown by: A. Glucose B. Fructose C. Sucrose D. Starch...
Mutarotation is shown by:
A. Glucose
B. Fructose
C. Sucrose
D. Starch
Solution
Some molecules when they dissolve in water exhibit the formation of α and β forms. Those exhibited α and β forms will be in equilibrium with each other. This change in rotation with respect to one other is called mutarotation.
Complete answer:
- In the question it is asked to find the molecule which is going to show the mutarotation among the given options.
- Generally, the molecule which contains free functional groups is going to mutarrotation.
- Carbohydrates are the best examples to show the mutarotation.
- Carbohydrates which have free aldehyde or ketone functional groups in their structures are going to show mutarotation in water.
- Coming to given options. Option A, Glucose. Glucose is an example for monosaccharide and contains a free hydroxyl functional group in its structure.
- The structure of the glucose is as follows.
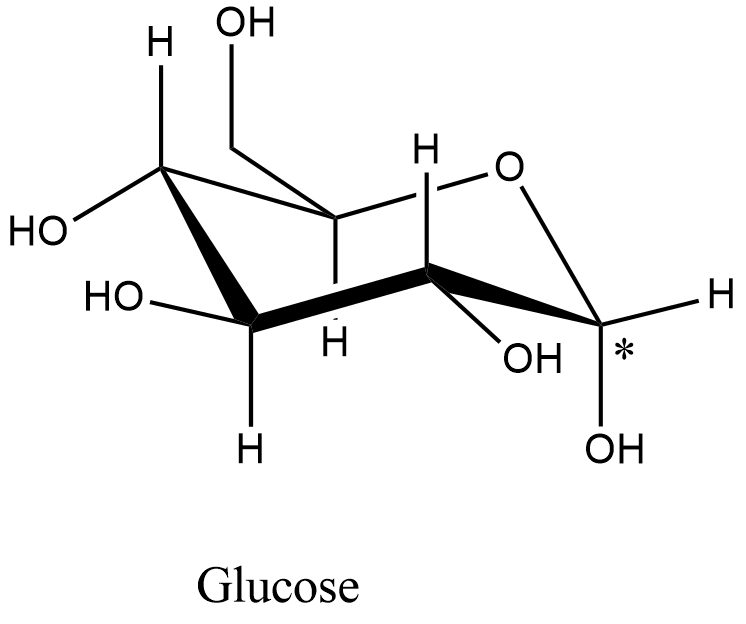
- The carbon with an asterisk symbol contains a free hydroxyl group and when the glucose is soluble in water the hydroxyl group is going to show the rotation.
- Therefore, glucose is going to show the mutarotation.
- Coming to option B, C, D. Fructose, Sucrose, Starch are the carbohydrates which are dimers of polymers (starch) in nature.
- The disaccharides or the polysaccharides do not show the mutarotation due to the absence of the free hydroxyl group, because the free hydroxyl group is going to be involved in the glycosidic bond to connect one monomer to another.
- Therefore, among the given options glucose is the only molecule which shows mutarotation.
So, the correct option is A.
Note:
The carbohydrate which is supposed to contain a free hydroxyl group to exhibit mutarotation when dissolved in water. If the hydroxyl group is going to be involved in the glycosidic bond then the molecule does not exhibit mutarotation.
