Question
Question: Multiple covalent bonds exist in a molecule of? Option- A.\({F_2}\) B.\({H_2}\) C.\({N_2}\) ...
Multiple covalent bonds exist in a molecule of?
Option-
A.F2
B.H2
C.N2
D.C2H6
Solution
Covalent bond is formed by the process of mutual sharing of valence shell electrons of combining atoms which may be the same or from different elements. Covalent bond is of three types: single bond, double bond or triple bond.
Complete answer:
Covalent bonds which are double bond or triple bond are collectively known as multiple covalent bonds because they contain more than one covalent bond. Double bond is formed between two atoms by the sharing of two electron pairs, and it is represented as (=). Triple bond formed between two atoms by sharing three electron pairs, and it is represented as (≡).
In the molecule of F2 two fluorine atoms are bonded with each other. As we know fluorine has seven electrons in its outermost shell and requires only one more electron to attain noble gas configuration neon. So, each fluorine atom shares one electron with each other to form a single bond.
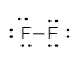
In the molecule of H2 two hydrogen atoms are bonded with each other. As we know hydrogen has one electron in its outermost shell and requires only one more electron to attain noble gas configuration of helium. So, each hydrogen atom shares one electron with each other to form a single bond.
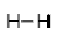
In the molecule of N2 two nitrogen atoms are bonded with each other. As we know nitrogen has five electrons in its outermost shell and requires three more electrons to attain noble gas configuration of neon. So, each nitrogen atom shares three electrons with each other to form a triple bond.

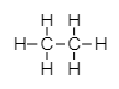
In the molecule of C2H6 two carbon atoms are bonded with each other. As we know carbon has four electrons in its outermost shell and requires four more electrons to attain noble gas configuration of neon. So, each carbon atom shares one electron with another carbon atom to form a single bond. And the remaining three electrons are shared with hydrogen atoms. C2H6 is a saturated organic compound with single covalent bonds only.
⇒ molecule of N2 contains multiple covalent bonds.
Therefore, option (C) is the correct option.
Note:
Double and triple bond easily undergoes the reaction of addition while single bond undergoes substitution mainly. Bond length of the atom decreases as we move from single bond to double bond and finally shortest to triple bond. However, the reactivity of the triple bond is maximum.
