Question
Question: Most stable sigma complex among the following is: A.
B.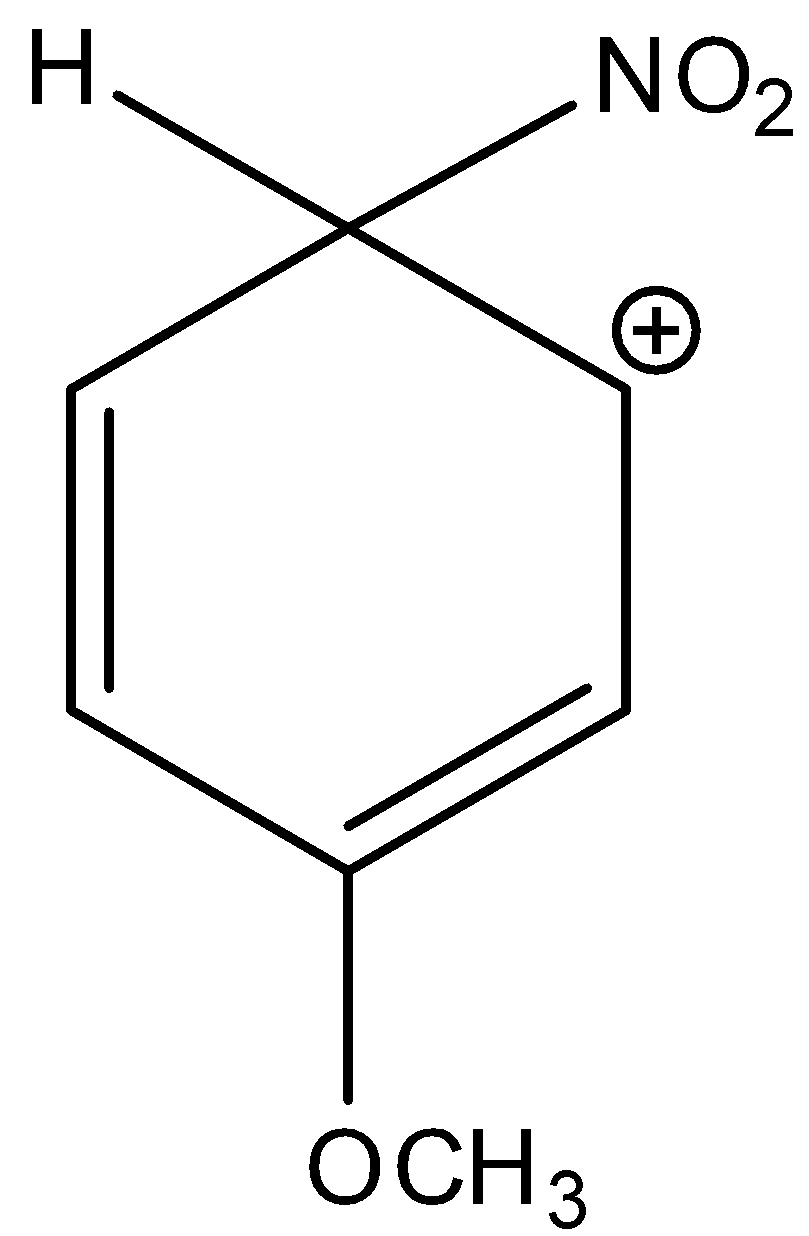
C.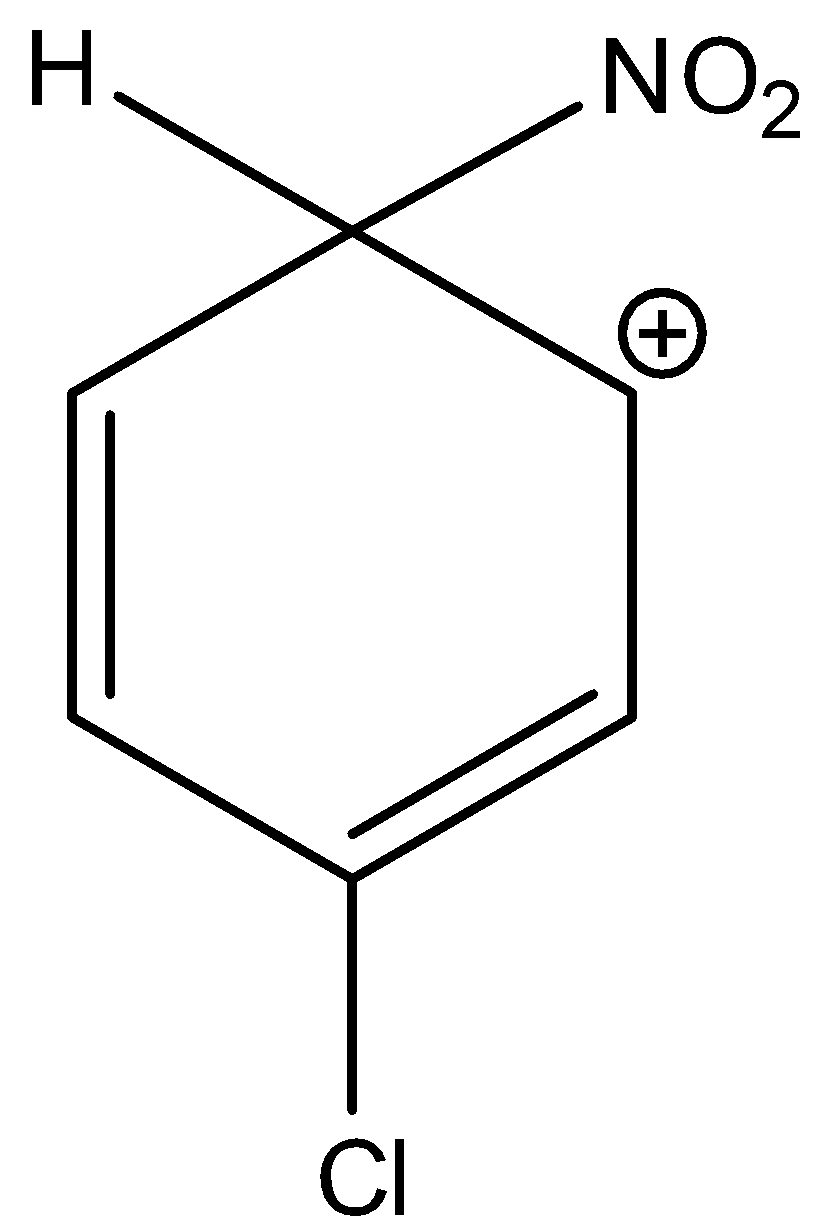
D.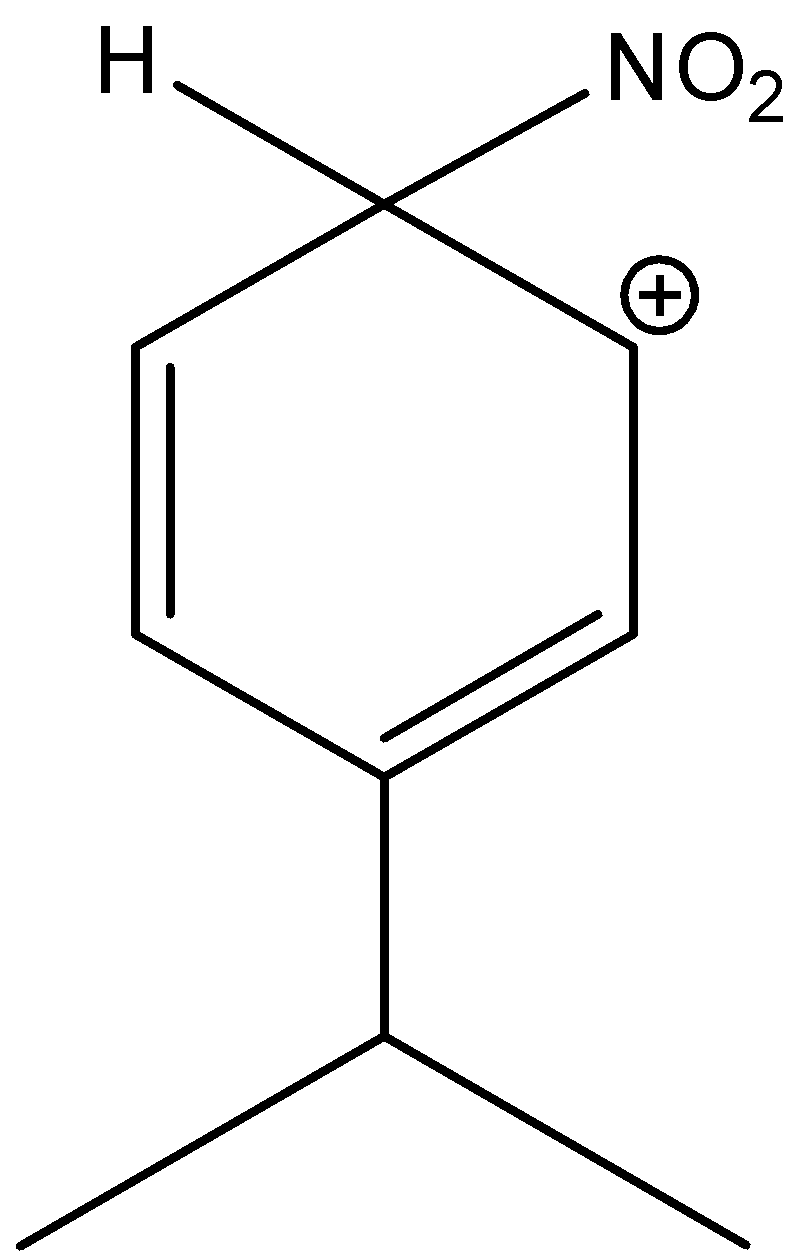
Solution
We need to know that the chemical stability is otherwise known as thermodynamic stability. And the stability of the compound depends on some factors like lattice energy, solvation energy, sublimation energy etc. And the stability also depends on the resonance structure of a compound and the size of the compound. If an electron withdrawing group is present in a compound, the compound becomes more stable.
Complete answer:
Here the complex is 3-methyl-6-nitrocyclohexa-2,4-dien-1-ylium. The methyl group is attached to the benzene ring. And it is not the most stable sigma complex. Hence, option (A) is incorrect.
Here, the given compound is 3-methoxy-6-nitrocyclohexa-2,4-dien-1-ylium. The methoxy group is attached in the third position of the benzene ring and the nitro group is also attached in this complex. And this methoxy group is ortho or para directing and an electron withdrawing group. There are two lone pairs present in the oxygen of methoxy group. Thus, it is more electronegative. And, it has a +M effect due to resonance. So, 3-methoxy-6-nitrocyclohexa-2,4-dien-1-ylium is a more stable sigma complex. Let’s see the resonance,
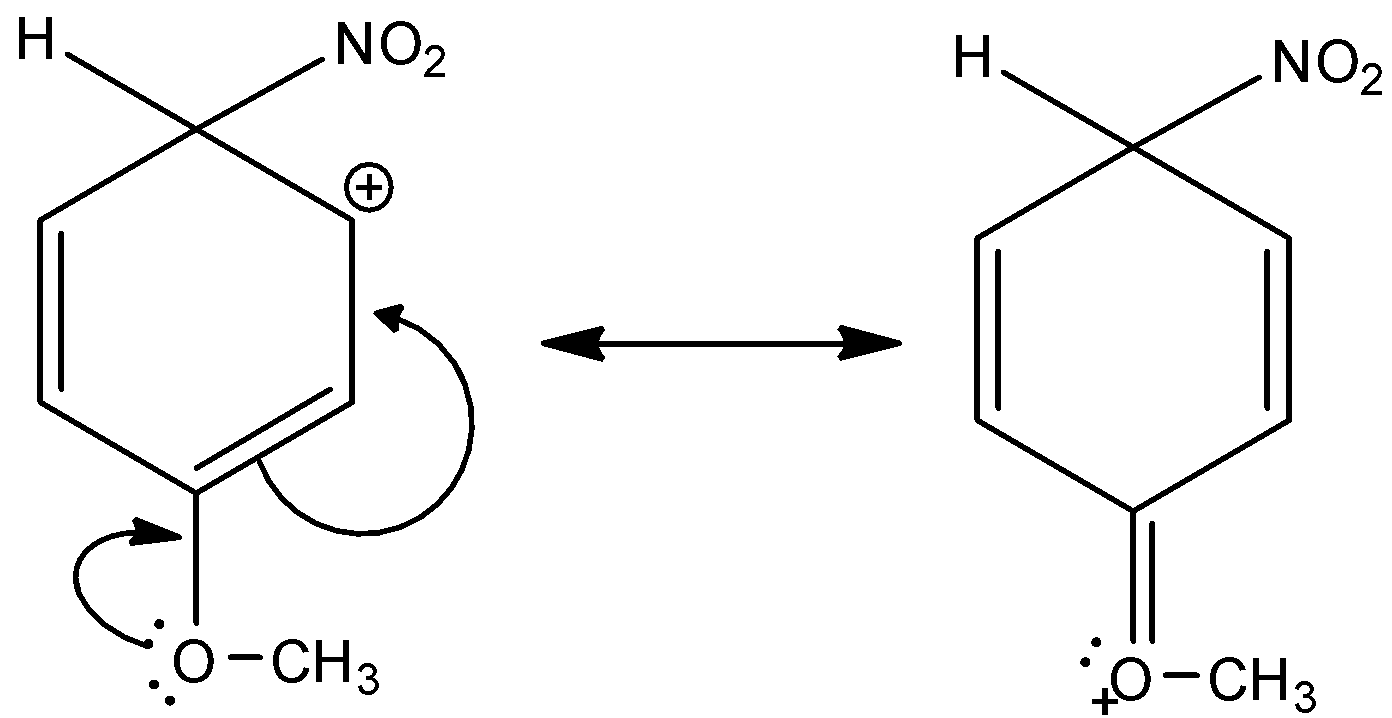
Hence, option (B) is correct.
3-chloro-6-nitrocyclohexa-2,4-dien-1-ylium is not a stable sigma complex. Hence, option (C) is incorrect.
3-isopropyl-6-nitrocyclohexa-2,4-dien-1-ylium is not the stable structure of the sigma complex. Hence, the option (D) is incorrect.
Hence, option (B) is correct.
Note:
If the nitration occurs at the ortho or para position, the sigma complex becomes more stable. The nitronium ion is reacting with the benzene and there is a formation of a sigma complex by the loss of a proton or hydrogen atom and an aromatic product. If there is a formation of a new sigma bond in the initial stage, that intermediate is known as the sigma complex.
