Question
Question: Most stable carbocation among the given example is: A. 
B. 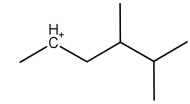
C. 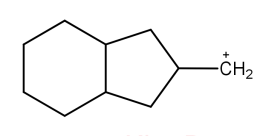
D. CH3+
Solution
Hint: Try to recall that there are many factors like dancing resonance, inductive effect, mesomeric effect, etc. on which the stability of carbocation. Now, by using this you can easily find the correct option from the given ones.
Complete step by step solution:
It is known to you that carbocation is a highly reactive intermediate species, they have one inherent positive charge and thus always in the lookout for electrons, which tells us about their reactivity.
So, the stabilization effects in carbocation are as follows:
Stability of carbocation is directly proportional to the +I effect, that is the reason tertiary carbocation is more stable than secondary (explained by hyperconjugation or dispersal of charge).
Carbocation stability increases with an increase in the percentage of p-character in hybridization.
Carbocation stability increases with increase with increasing alpha hydrogen atoms due to hyperconjugation.
Carbocations are stabilized by neighboring carbon-carbon multiple bonds due to resonance.
A carbocation is stabilized by adjacent lone pairs of the electron due to resonance.
Now, coming to the question all the four molecules given in option get stabilized by hyperconjugation. Alpha hydrogen is that hydrogen that is attached to alpha carbon (first carbon atom that attaches to a functional group). That carbocation will be more stable which will have the maximum number of alpha hydrogen. From all options, option A has the most number of alpha hydrogen (7) and will be the most stable carbocation.
Therefore, from above we can say that option A is the correct option to the given question.
Note: It should be remembered to you that dancing resonance is a hypothetical phenomenon that is shown in structures of carbocation attached directly to a three-membered ring.
Also, you should remember that aromaticity is the property of a molecule’s structure by which it gains additional stability due to its geometry and makes the compound less reactive.
