Question
Question: More the power of –I effect more will be acidity (opposite for +I effect). On the basis of the given...
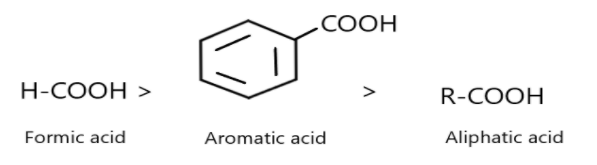
More the power of –I effect more will be acidity (opposite for +I effect). On the basis of the given statement check whether the given order is correct or not?
Solution
We have already learnt that the inductive effect is a permanent effect that arises whenever an electron withdrawing group is attached to the carbon chain. This effect weakens with increasing distance from the substituent and becomes almost negligible after three carbon atoms.
Complete answer:
We know that the inductive effect arises when an electron withdrawing group (EWG) is attached to the carbon chain. For example- let us assume a carbon chain with chlorine attached at one end C4−C3−C2−C1−Cl. Since chlorine is more electronegative than carbon, the σ-electrons of C−Cl bond are attracted towards chlorine and it acquires a small negative charge and C1 acquires a small positive charge which will attract the σ-electrons of the other carbon atoms resulting in the more positive charge on carbon atoms and hence the displacement of these σ-electrons along a saturated carbon chain whenever an EWG is present is the inductive effect or I-effect.
This effect weakens with increasing distance from the substituent and becomes almost negligible after three carbon atoms. There are two types of inductive effects:
(a) −Ieffect and
(b) +Ieffect
−Ieffect involves the permanent shifting of σ-electrons away from carbon atoms due to an atom or a group.
Example: −NO2>−CN>−COOH>−F>−Cl>−Br>−I>−OH
+Ieffect involves the permanent shifting of σ-electrons towards the carbon atom due to an atom or a group.
Example: (CH3)3C−>(CH3)2CH−>CH3−CH2−>CH3
Groups that have +Ieffect increases the electron density on the molecule responsible for donating an electron and becomes basic whereas groups with−Ieffect decreases the electron density and molecules becomes acidic thereby becoming electron deficient.
As the number of −Igroups increases, acidity also increases, opposite is the case of +Igroups.
Thus, the given order is correct as the formic acid is more stable and acidic due to stability of conjugate base whereas benzoic acid is stabilised due to resonance of ring structure and aliphatic acid possesses less acidity than the other two.
Note:
Inductive effect is responsible for the high melting point, high boiling point and greater dipole moment of polar compounds. This effect weakens with increasing distance from the substituent and becomes almost negligible after three carbon atoms, this is the key point to keep in mind about inductive effect.
