Question
Question: Mononitration of phenyl methanoate....
Mononitration of phenyl methanoate.
Solution
Formate whose IUPAC name is methanoate is the anion derived from formic acid. Its formula is chloroformate, CH3OCOCl. The combined influence of —OH and —CH3 groups determines the position of the incoming group.
Complete answer:
Phenyl methanoate is a compound having very less shelf- life. So, it is very unstable. Its formula is C6H5OCOH.
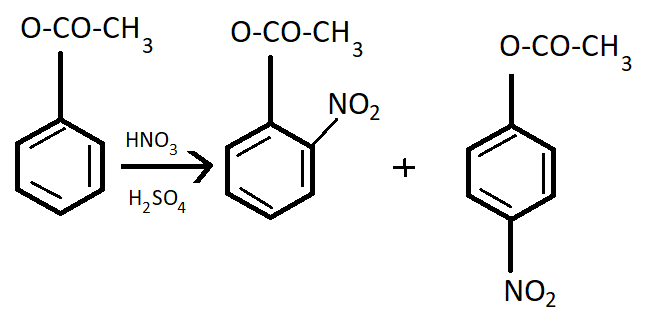
It goes to the para position because the Benzene ring will be unstable if the NO2group is attached anywhere else. It is an electron donating group and also an unstable compound at Meta or Ortho positions.
This process occurs in the presence of HNO3 and H2SO4which is the nitrating mixture.
Note: Phenyl methanoate is a compound having very less life and hence it is very unstable. Its nitration is not feasible to carry out. But still if nitration is carried out then it will give a meta product. In such types of problems, the properties of compounds are very important.
