Question
Question: Monomer of polypropene/polypropylene is: A. propane B. propene C. propyne D. prop-1,2-diene...
Monomer of polypropene/polypropylene is:
A. propane
B. propene
C. propyne
D. prop-1,2-diene
Solution
We know that polymer is a huge compound formed from joining of repeating units (monomers) in a large scale. This process of polymer formation from monomers is coined as polymerization. Polyethene is the common polymer that we use in our everyday life.
Complete step by step answer:
Polypropene is a polymer whose structure is,
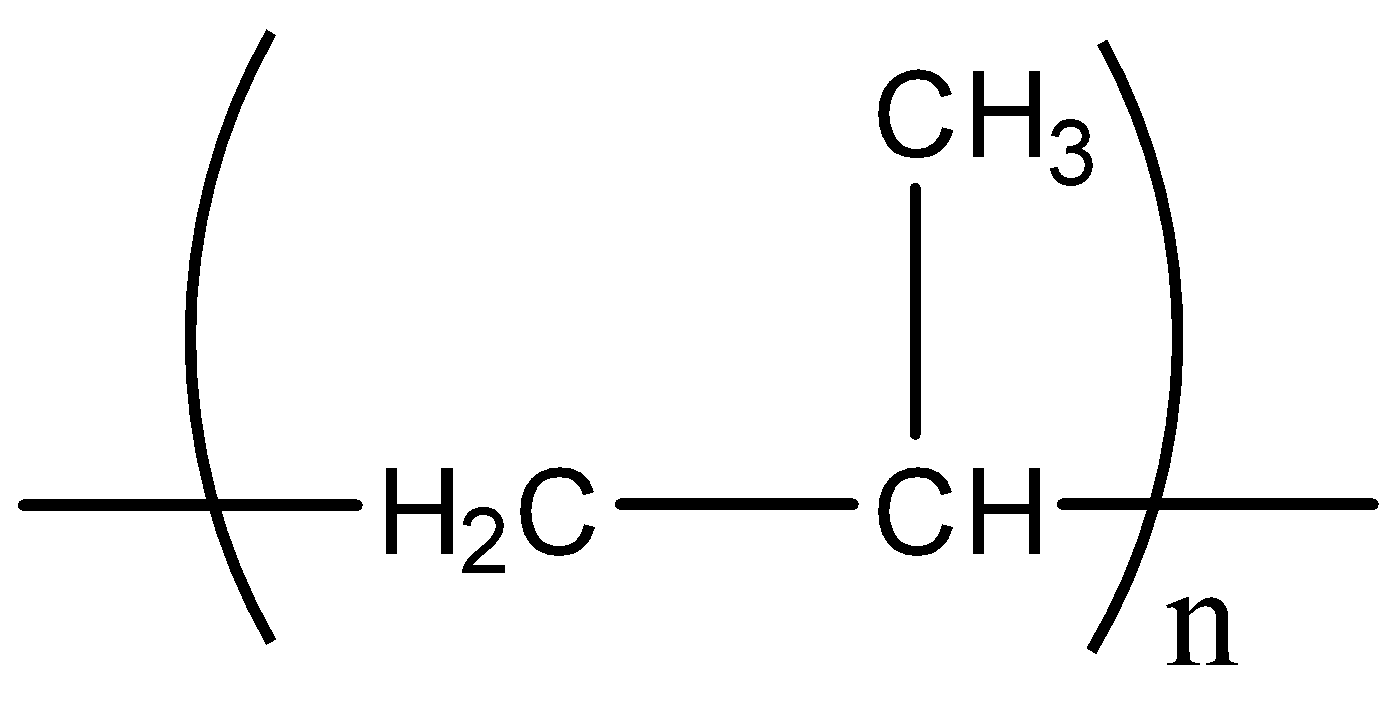
Polypropene can be formed by reaction of n number propene in presence of Ziegler-Natta catalyst.
The reaction can be shown as below:
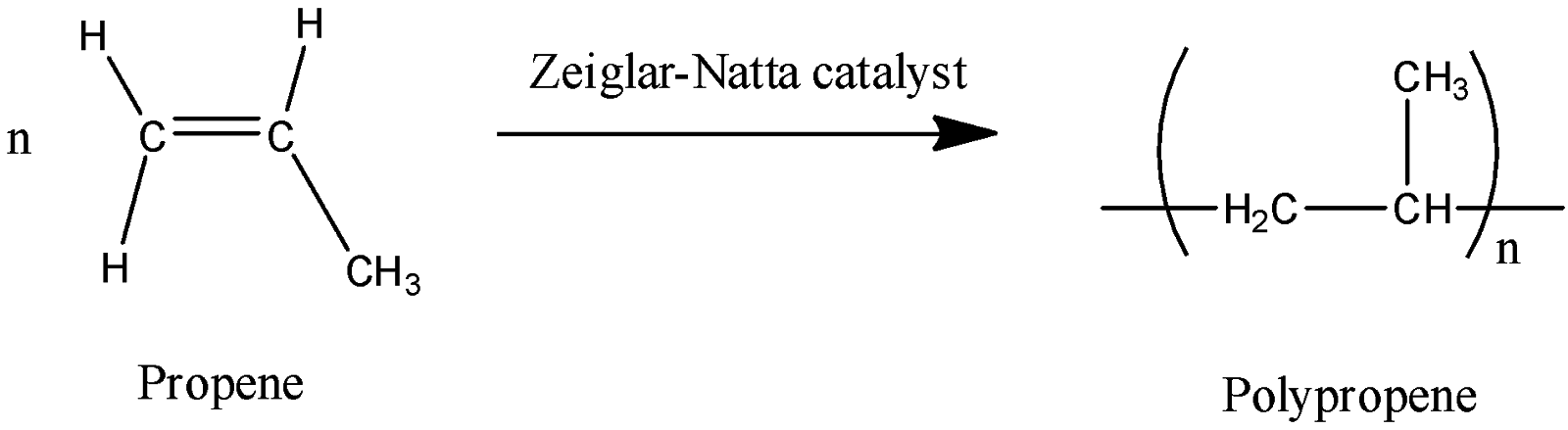
From the above reaction, it is confirmed that monomer of polypropene is propene.
So, the correct answer is “Option B”.
Additional Information: The polymers are classified on the basis of its source, its structure, its mode of polymerization etc.
-On the basis of its source polymers are classified as natural polymer, semisynthetic polymer and synthetic polymer.
-Natural polymers are those polymers which are found in animals and plants, such as, protein, cellulose, rubber, starch, etc.
-Semi synthetic polymers are the polymers mostly obtained by chemical modifications of naturally occurring polymers. Some examples are vulcanized rubber, rayon, etc.
Synthetic polymers are the polymers which are made in factories, such as, Buna-S, Buna-N etc.
-There are two kinds of polymerization reactions, addition polymerization and condensation polymerization. In addition polymerization, same or different monomers joined together to result a polymers such as in case of formation of polyethene and in condensation polymerization, repetitive condensation of two bi-functional monomers occurs to form polymers, such as in case of formation of nylon 6,6.
Note: Polypropene is the polymer which is formed by addition polymerization. It is an example of thermoplastic polymer. It is used in the manufacture of pipes, ropes, toys, fibres etc. It possesses properties similar to polyethene but it is harder and more heat resistant than polyethene.
