Question
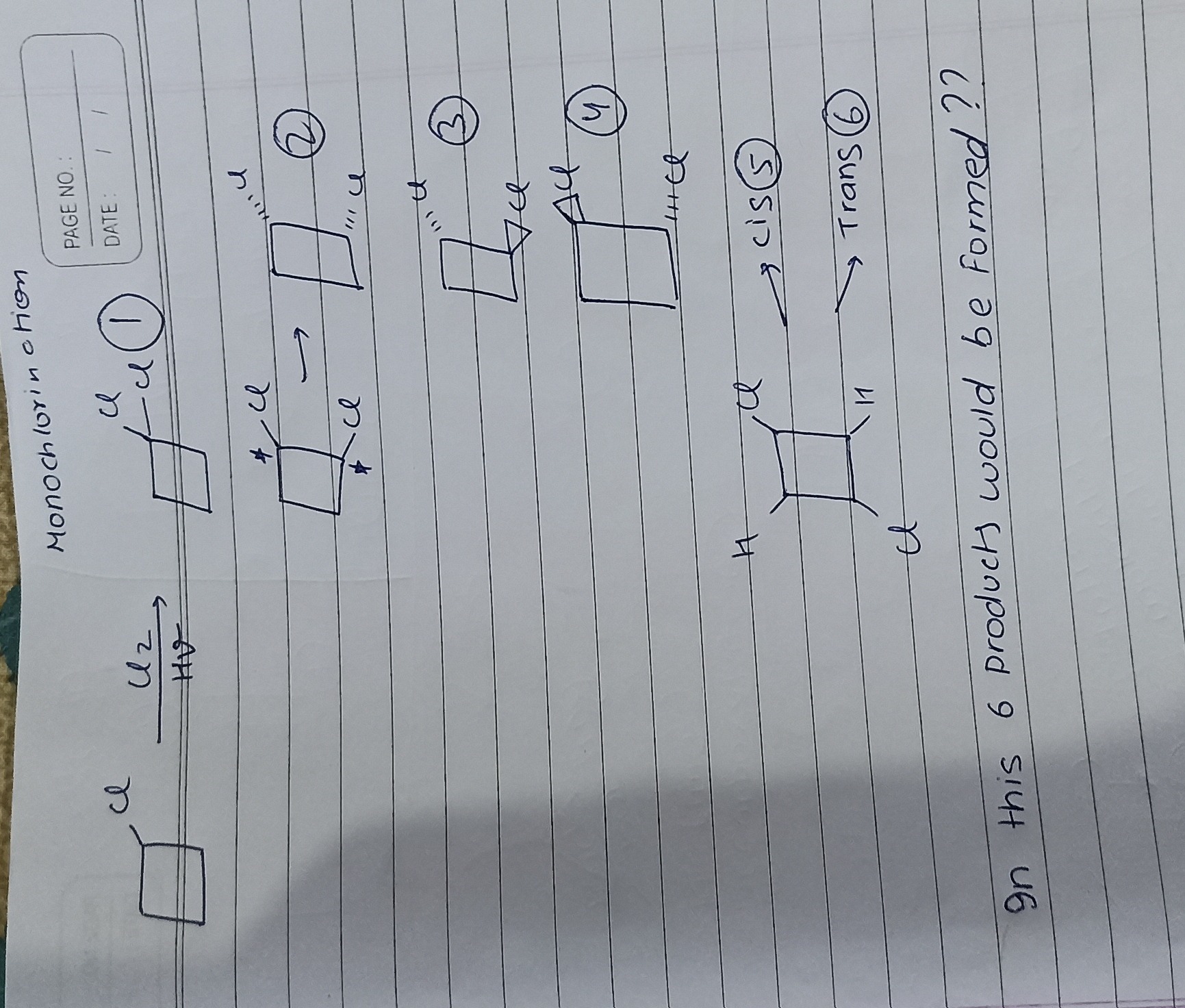
Question: In this 6 products would be formed ??...
In this 6 products would be formed ??
True
False
6
Solution
The question asks about the number of monochlorination products formed from chlorocyclobutane. Monochlorination is a free radical substitution reaction where one hydrogen atom is replaced by a chlorine atom.
Let's analyze the structure of chlorocyclobutane and identify the different types of hydrogen atoms available for substitution. We number the carbon atoms starting from the carbon bearing the chlorine atom.
Due to the symmetry of the cyclobutane ring, C2 and C4 are equivalent positions. Therefore, we have three distinct positions where a hydrogen atom can be replaced by a chlorine atom:
- C1 (the carbon already bearing a chlorine atom): This carbon has one hydrogen atom.
- C2 (or C4, which are equivalent): These carbons each have two hydrogen atoms.
- C3 (the carbon opposite to C1): This carbon has two hydrogen atoms.
Let's analyze the products formed by chlorination at each position:
1. Chlorination at C1: Replacing the hydrogen at C1 leads to 1,1-dichlorocyclobutane.
This molecule has a plane of symmetry passing through C1 and C3, and bisecting the C2-C4 bond. Therefore, it is achiral.
- Number of products from C1 chlorination: 1
2. Chlorination at C2 (or C4): Replacing a hydrogen at C2 leads to 1,2-dichlorocyclobutane. This molecule has two chiral centers (C1 and C2). Therefore, it can exist as stereoisomers (cis/trans and enantiomers).
-
cis-1,2-dichlorocyclobutane: The two chlorine atoms are on the same side of the cyclobutane ring.
This molecule has a plane of symmetry (passing through the midpoints of the C1-C2 and C3-C4 bonds, and perpendicular to the ring). It is a meso compound and therefore achiral.
- Number of products: 1
-
trans-1,2-dichlorocyclobutane: The two chlorine atoms are on opposite sides of the cyclobutane ring.
This molecule is chiral. It does not possess any plane of symmetry or center of inversion. Therefore, it exists as a pair of enantiomers (e.g., (1R,2S) and (1S,2R)).
- Number of products: 2
-
Total products from C2/C4 chlorination: 1 (cis, meso) + 2 (trans, enantiomers) = 3
3. Chlorination at C3: Replacing a hydrogen at C3 leads to 1,3-dichlorocyclobutane. This molecule also has two chiral centers (C1 and C3).
-
cis-1,3-dichlorocyclobutane: The two chlorine atoms are on the same side of the cyclobutane ring.
This molecule has a plane of symmetry passing through C1 and C3, and perpendicular to the ring. It is a meso compound and therefore achiral.
- Number of products: 1
-
trans-1,3-dichlorocyclobutane: The two chlorine atoms are on opposite sides of the cyclobutane ring.
This molecule has a plane of symmetry passing through C2 and C4, and perpendicular to the ring. It is also achiral.
- Number of products: 1
-
Total products from C3 chlorination: 1 (cis, achiral) + 1 (trans, achiral) = 2
Total number of unique monochlorination products: Summing the products from each position: 1 (from C1) + 3 (from C2/C4) + 2 (from C3) = 6 products.
The products are:
- 1,1-dichlorocyclobutane (achiral)
- cis-1,2-dichlorocyclobutane (meso, achiral)
- trans-1,2-dichlorocyclobutane (chiral, pair of enantiomers)
- cis-1,3-dichlorocyclobutane (meso, achiral)
- trans-1,3-dichlorocyclobutane (achiral)
The question asks "In this 6 products would be formed ??". Based on the analysis, yes, 6 products would be formed.
