Question
Question: Monoatomic gas is kept under a piston of mass 3 kg in a vessel in equilibrium. Vessel & piston are t...
Monoatomic gas is kept under a piston of mass 3 kg in a vessel in equilibrium. Vessel & piston are thermally insulated. Piston can move vertically without friction. A block of mass 5 kg is put on top of piston and allowed to attain the equilibrium as in figure (b). The temperature of gas becomes T1. After removing the block piston moves upward and when equilibrium is attained again temperature of gas becomes T2. Neglect atmospheric pressure. Then-
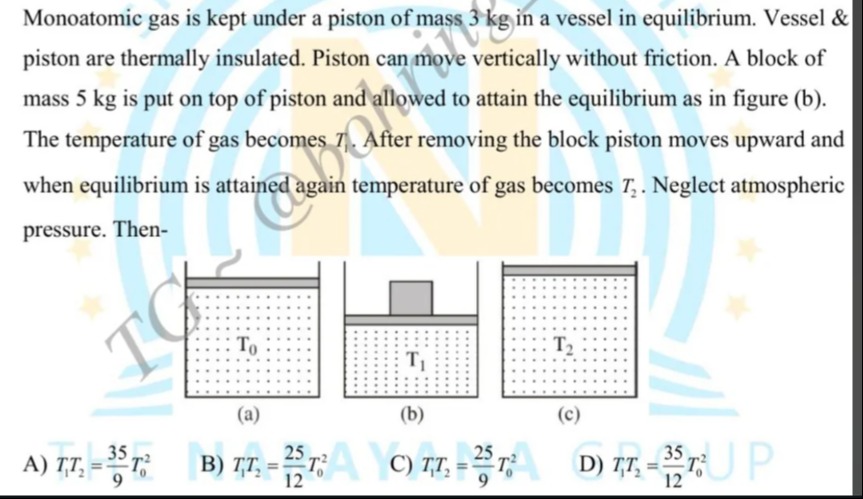
T1T2=935T02
T1T2=1225T02
T1T2=925T02
T1T2=1235T02
None of the above. The correct relationship is T1T2=T02(38)2/5
Solution
The system undergoes adiabatic processes. First, a 5 kg block is added, compressing the gas and raising the temperature from T0 to T1. Then, the block is removed, allowing the gas to expand and cool to T2.
Key relationships:
-
Adiabatic process: TγP1−γ=constant, where γ=35 for a monoatomic gas. This implies T∝Pγ1−γ which simplifies to T∝P2/5.
-
Pressure: Pressure is determined by the weight on the piston divided by its area (A).
- P0=A3g
- P1=A8g
- P2=A3g
Step 1: Process from state (a) to (b)
T0T1=(P0P1)2/5=(3g/A8g/A)2/5=(38)2/5 T1=T0(38)2/5
Step 2: Process from state (b) to (c)
T1T2=(P1P2)2/5=(8g/A3g/A)2/5=(83)2/5 T2=T1(83)2/5
Step 3: Calculate T1T2
T1T2=T0(38)2/5×T1(83)2/5=T0T1(38)2/5(83)2/5=T0T1
Substitute T1:
T1T2=T0(38)2/5T0=T02(38)2/5
Therefore, T1T2=T02(38)2/5. None of the provided options match this result, suggesting a potential error in the question or answer choices.
