Question
Question: Molybdenum forms body-centred cubic crystals whose density is 10.3\(g.c{m^{ - 3}}\). Calculate the e...
Molybdenum forms body-centred cubic crystals whose density is 10.3g.cm−3. Calculate the edge length of the unit cell. The molar mass of Mo is 95.94g.mol−1.
Solution
First find out the total number of atoms present in a bcc lattice. Then use the formula: d=NA×a3z×M to calculate the edge length of the unit cell, which can also be written as:
a=3NA×dz×M
Formula used:
-Density of a unit cell: d=NA×a3z×M (A)
Where, d = density of the unit cell;
z = number of atoms present in the unit cell;
M = molar mass of the atom;
NA= Avogadro number;
a = side length of the unit cell.
Complete answer:
-First of all we need to see what a body centred cubic crystal (bcc) is.
In a body centred cubic unit cell or crystal atoms are present at each of its 8 corners and one atom is present at the body centre.
The total number of atoms present in a bcc crystal are:
(1)8 corner atoms × (1/8) contribution of each corner atom = 1 atom
(2)1 body centre atom
So, in total 2 atoms are present in a bcc crystal. It looks like:
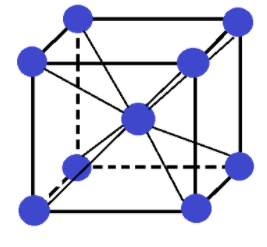
-The values for a Mo bcc structure given in the question are: d = 10.3g.cm−3, M = 95.94g.mol−1 and we know that NA= 6.023×1023. Also in the above discussion we just saw that for a bcc crystal z = 2.
Since we have d, M, NAand z; we can find out the value of ‘a’ using the equation (A).
d=NA×a3z×M
10.3=6.023×1023×a32×95.94
a3=6.023×1023×10.32×95.94
= 62.0369×1023191.88
= 3.092997×10−23cm3
a=33.092997×10−23
= 3.14×10−8cm
= 3.14 Å
So, the side of the cubic crystal is 3.14 Å.
Note:
In a bcc crystal or lattice there are only 2 atoms present and so it has a packing efficiency of 68%. Iron, chromium, tungsten and niobium also exhibit bcc structure.
