Question
Question: Molecular weight of water is 20. A. True B. False...
Molecular weight of water is 20.
A. True
B. False
Solution
Molecular weight is the weight of each atom given in atomic mass. Atomic mass is the total number of protons and neutrons in the nucleus of an atom and is represented by the ‘A’. Nucleus and protons together are called nucleons. For example, the carbon atom has 6 protons and 6 neutrons and its atomic number is 12. The number of neutrons in an atom may vary but the number of protons remains the same.
The molecular weight is used to tell how many grams of that molecule is present in 1 mole of that chemical substance.
Complete step by step answer:
The chemical formula of water is H2O which contains two hydrogen atoms and one oxygen atom.
Let us calculate the molecular weight of water.
H2O
=2×1+16
=18
(The two hydrogen has an atomic mass of 1 and the atomic mass of oxygen atom is 16 and after calculating we get the molecular weight of water which is found to be 18).
So, the statement is false.
Note: Water was considered to be an element until 1781. However, Cavendish in 1781, showed that it can be obtained by burning hydrogen. Therefore, it is not an element. Lavoisier (1781) confirmed the compound nature of water. Davy (1800) showed that the water is composed of hydrogen and oxygen in the ratio 2:1 by volume. Dumas (1842) established that water contains hydrogen and oxygen in the ratio 1:8 by weight.
The structure of the water molecule is
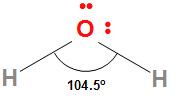
The H−O−H bond angle in water is 104.5∘ and the O−H bond length is 95.7 pm. Because of the high electronegativity difference between oxygen and hydrogen, the O−H bonds in water are polar. The oxygen due to high electronegativity has a partial negative charge and the hydrogen atoms due to their lowest electronegativity have partial positive charges. The dipole moment of water is 1.84 D which confirms its polar nature.
