Question
Question: Molecular formula of chlorine is \(C{l_2}\). Draw electron-dot and line structure of a chlorine mole...
Molecular formula of chlorine is Cl2. Draw electron-dot and line structure of a chlorine molecule.
Solution
Chlorine atoms have seven electrons in its valence shell and it needs only one electron to combine to complete its octet. In electron dot structure we represent electrons and in line structure we just represent the shared electron pair by a line.
Complete step by step answer:
As we know that chlorine is a halogen atom and it belongs to group number 17. Chlorine has atomic number 17. Chlorine atom has seven valence electrons. The most of the atoms combined according to the octet rule. The octet rule is a chemical rule according to which the main group elements combine to form bonds in such a way that each atom will have eight electrons in its valence shell to get the same electronic configuration as that of the corresponding noble gas configuration. This is because noble gases are the most stable elements of the periodic table and each element wants to get stable.
As we know that chlorine has seven electrons in its valence shell and according to the octet rule chlorine will tend to bond in such a way that it will have eight electrons in its valence shell. Therefore, chlorine shares one electron with another atom of chlorine so that both the chlorine will have eight electrons in its valence shell.
In electron dot structure we represent each electron by a dot. According to it, Cl2 will have given electron dot structure:
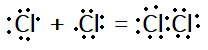
In line structure, we will represent only the shared pair of electrons with a line. For Cl2, it can be given as:
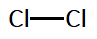
Note:
Always remember that there are some elements which do not follow octet rule such as in case of aluminium, boron, etc. As in BF3, boron readily forms compounds in which they have six valence electrons rather than usual eight electrons according to octet rule.
