Question
Question: Molecular formula of benzoic acid is: A.) \[{{C}_{6}}{{H}_{5}}COOH\] B.) \[{{C}_{6}}{{H}_{6}}COO...
Molecular formula of benzoic acid is:
A.) C6H5COOH
B.) C6H6COOH
C.) C5H6COOH
D.) None
Solution
Hint: We know that benzoic acid is an acid and it is an organic compound. From the benzo word we can understand that it is a compound of benzene. And from the acid word we can understand that it has H+ ions.
Step by step solution:
Benzoic acid is an acid and can release H+ ion means hydrogen attached with a more electronegative element or group. Benzoic acid has six carbons, five hydrogens and one carboxylic acid group with it. So the chemical formula of benzoic acid is: C6H5COOH
And its chemical structure is:
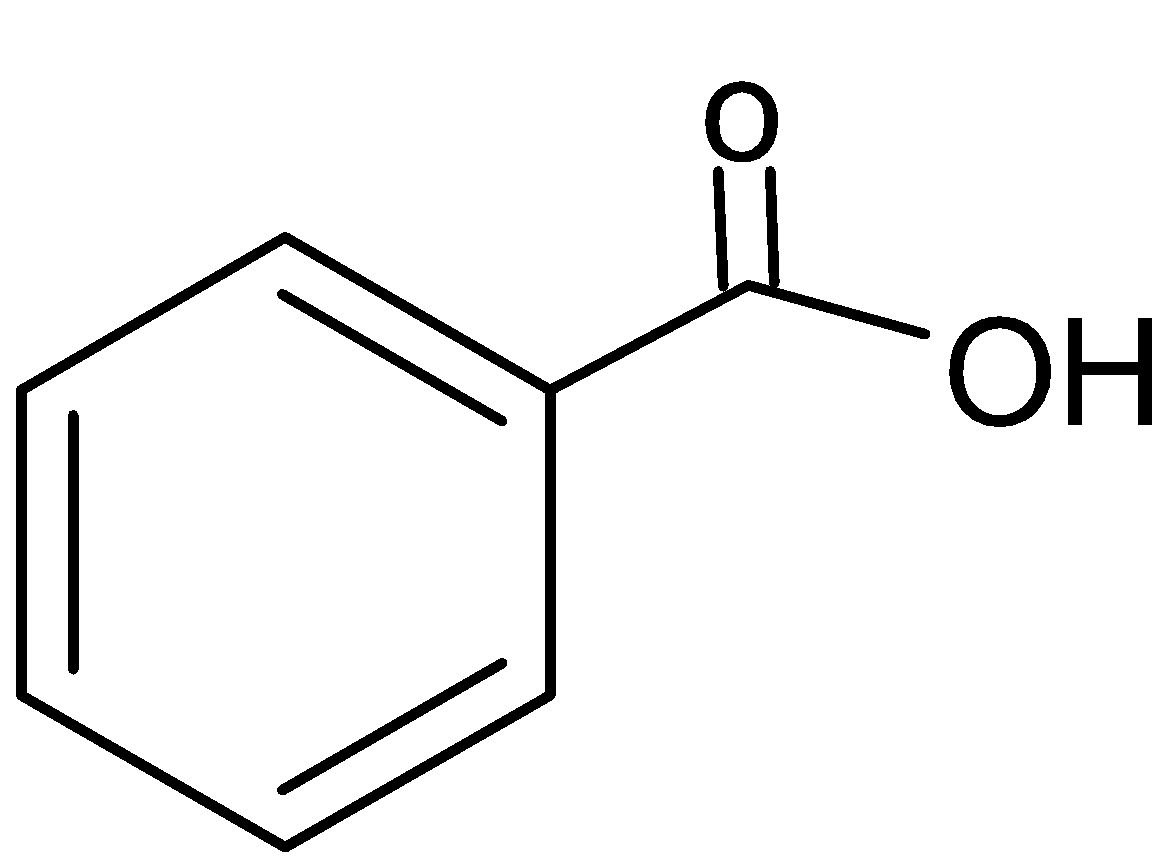 It is classified as an aromatic carboxylic acid. It’s aromatic due to the presence of a benzene ring in its chemical structure. And it’s a carboxylic acid due to the presence of carboxyl group (−COOH) in its structure. This acid is most commonly found in industrial settings to manufacture a wide variety of products such as perfumes, dyes etc.
It is classified as an aromatic carboxylic acid. It’s aromatic due to the presence of a benzene ring in its chemical structure. And it’s a carboxylic acid due to the presence of carboxyl group (−COOH) in its structure. This acid is most commonly found in industrial settings to manufacture a wide variety of products such as perfumes, dyes etc.
So, from the above explanation we can say that option “A” is the correct answer.
Option “B” is incorrect because it has extra hydrogen in it which doesn’t fulfill the valency rule of carbon or benzene.
Option “C” is incorrect because it is a 5 membered ring which is not a benzene ring.
Note: Here “ic” suffix is for carboxylic acid and benzo is for benzene. So, you should be careful while taking the acidic group. This Compound neutralizes the basic medium, which tells that it is an acid. It is also known as benzenecarboxylic acid.
