Question
Question: Molar mass of glucose is: (A) 180g (B) 129g (C) 190g (D) 200g...
Molar mass of glucose is:
(A) 180g
(B) 129g
(C) 190g
(D) 200g
Solution
To answer this question firstly we should know the chemical formula of glucose and secondly, the definition as well as the formula to calculate molar mass. This will help us in solving the question within no time.
Complete answer:
The glucose is a monosaccharide, that is found in free State in fruits and in some parts of the plants. It contains an aldehyde group and a six carbon atom.
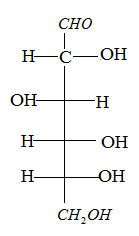
The chemical formula is C6H12O6.
Molar mass is the arithmetic sum of atomic mass of elements in the molecule.
Molar mass = 1 moleMolecular weight in gram
The mass of a particular atom of an element which is expressed in atomic mass units.
The atomic mass of carbon = 12
The atomic mass of hydrogen =1.008
The atomic weight of oxygen = 16
The molar mass of glucose = atomic mass of carbon×6 + atomic mass of hydrogen×12 + atomic mass of oxygen × 6
Any number divided by 1 gives the same number.
= 12 × 6 + 1.008 ×12 + 16 × 6
= 180.096g
So, from the above discussion we can conclude that option A is the correct answer.
Note:
It is very important to be careful while calculating. It is better to remember the atomic mass of elements in periodic tables. If we know the atomic mass of the elements and formula to calculate in hand, solving this question takes less time.180g means there is 180g per mole as the definition molar mass is measured in grams per mole. As we know that any number divided by 1 gives the same number.
