Question
Question: Molar conductivity (\[ \wedge m\]) of aqueous solution of sodium stearate, which behaves as a strong...
Molar conductivity (∧m) of aqueous solution of sodium stearate, which behaves as a strong electrolyte, is recorded at varying concentrations (c) of sodium stearate. Which one of the following plots provides the correct representation of micelle formation in the solution? (critical micelle concentration (CMC) is marked with an arrow in the figures)
Option:
A.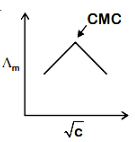
B.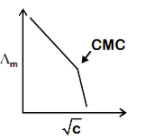
C.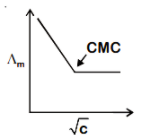
D.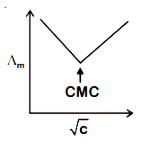
Solution
Sodium stearate has both hydrophilic and hydrophobic components, the carboxylate and the long hydrocarbon chain, which are typical of soaps. These two chemically distinct components cause micelles to form, with hydrophilic heads facing outwards and hydrophobic (hydrocarbon) tails facing inwards, resulting in a lipophilic atmosphere for hydrophobic compounds. The micelle is formed when the grease (or dirt) is dissolved by the tail portion. It's also used as a surfactant in the pharmaceutical industry to help with the solubility of hydrophobic materials in the manufacture of mouth foams
Complete answer:
Micelles are lipid molecules that form spherical structures in aqueous solutions. Micelle formation is a reaction of fatty acids' amphipathic composition, which means they have both hydrophilic (polar head groups) and hydrophobic (polar tail groups) areas (the long hydrophobic chain).
Molar conductivity is known as the conductivity of an electrolyte's solution divided by the electrolyte's molar concentration, and it measures how efficiently an electrolyte conducts electricity in solution.
Sodium stearate →CH3(CH2)16COO−Na+
Stearate ions get clubbed together and form micelles as the concentration of sodium stearate rises above CMC. The concentration of current carrier anions drops sharply as a result of this. The sharp shift in ∧m at CMC, accompanied by a faster rate of ∧m decrease with CCMC, reflects this.
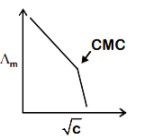
Hence option B is correct.
Note:
The essential micelle concentration (CMC) is defined in colloidal and surface chemistry as the concentration of surfactants above which micelles form and any additional surfactants added to the system form micelles.
A surfactant's CMC is an essential trait. The surface tension varies dramatically with surfactant concentration before hitting the CMC. The surface tension stays largely stable or shifts with a lower slope after exceeding the CMC. Temperature, heat, and (sometimes strongly) the presence and concentration of other surface active substances and electrolytes all influence the CMC for a given dispersant in a given medium.
