Question
Question: Molality of pure liquid benzene $(C_6H_6)$ if its density $d = 2$ gram/ml is -...
Molality of pure liquid benzene (C6H6) if its density d=2 gram/ml is -
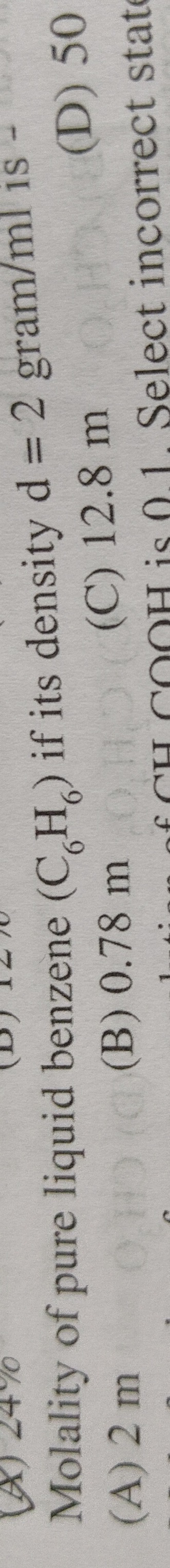
2 m
0.78 m
12.8 m
50 lution of CH3COOH is 0.1, Select incorrect state
12.8 m
Solution
To calculate the molality of a pure liquid, we consider the substance itself as both the solute and the solvent.
Molality (m) is defined as the number of moles of solute per kilogram of solvent.
m=mass of solvent (in kg)moles of soluteFor a pure liquid like benzene (C6H6), let's consider a sample of 1 kilogram (1000 grams) of the liquid. In this context, this 1 kg of benzene acts as the 'solvent'. The 'solute' would then be the moles of benzene present in this 1 kg.
Step 1: Determine the molar mass of benzene (C6H6).
The atomic mass of Carbon (C) is approximately 12.01 g/mol.
The atomic mass of Hydrogen (H) is approximately 1.008 g/mol.
Molar mass of C6H6=(6×Atomic mass of C)+(6×Atomic mass of H)
Molar mass of C6H6=(6×12.01 g/mol)+(6×1.008 g/mol)
Molar mass of C6H6=72.06 g/mol+6.048 g/mol=78.108 g/mol
For practical purposes in competitive exams, often rounded values are used:
Molar mass of C6H6≈(6×12)+(6×1)=72+6=78 g/mol.
Step 2: Calculate the number of moles of benzene in 1 kg (1000 g).
Moles of benzene = Molar mass of benzeneMass of benzene
Moles of benzene = 78 g/mol1000 g≈12.82 mol
Step 3: Calculate the molality.
Since we considered 1 kg of benzene as the 'solvent', and the moles of benzene in it are 12.82 mol, the molality is:
Molality = 1 kg12.82 mol=12.82 m
The density of benzene (d = 2 gram/ml) given in the question is a distractor. For the molality of a pure liquid, the density is not required as it cancels out if we consider any arbitrary volume, or it is not used if we directly consider 1 kg of the substance.
For example, if we consider a volume V (in mL) of pure benzene:
Mass of benzene = V×d (in grams)
Moles of benzene = MV×d
Mass of solvent (in kg) = 1000V×d
Molality = Mass of solvent (in kg)Moles of benzene=(V×d)/1000(V×d)/M=MV×d×V×d1000=M1000
As shown, the density (d) and volume (V) cancel out.
The calculated molality is approximately 12.8 m.
