Question
Question: MnO is? A. ferromagnetic B. antiferromagnetic C. ferrimagnetic D. diamagnetic...
MnO is?
A. ferromagnetic
B. antiferromagnetic
C. ferrimagnetic
D. diamagnetic
Solution
The electronic configuration of the element is responsible for the characteristics asked in the question. Manganese has the maximum number of the unpaired electrons in its excited state. It also has a half-filled configuration in its 3d subshell which decides the nature of magnetism in its oxide.
Complete step by step solution:
-After a lot of experiments, atomic structure was proposed and the probability of electrons were found to be maximum in a space known as orbital. Based on further studies, 4 quantum numbers were proposed which were able to give a complete picture of the electron location.
-Principle quantum number represents the shell of the electrons. It is represented by n. It gives a complete picture of shells and also the number of sub-shells. Value of n is always in natural numbers. First shell is denoted as K, second as L, third as M and fourth as N. Number of subshells is given by value of n and of orbitals by n2 .
-Next is azimuthal quantum number denoted as l. It tells about the subshell and the number of orbitals. It starts from 0 and continues till (n-1). It also gives us the shape of the orbitals. The number of orbitals lie in the range from (-l to +l) as integers.
-Third quantum number is magnetic quantum number and is denoted by m. It gives us the exact orbitals. Its total value can be given as n2 or as (2l+1) since it lies in the range (-l to +l).
-Last quantum number is spin quantum number which tells us about the spin of an electron in the orbital. It is denoted as s. It has only 2 values, +0.5 and -0.5. If the orientation is clockwise, then the spin is positive or negative.
-Spin of an atom decides its magnetic behavior. If the electrons are paired, the atom is diamagnetic else paramagnetic which is divided into ferromagnetism, ferrimagnetism and antiferromagetism.
-When the spins of electrons are parallel to the magnetic field, the atom is ferromagnetic. When spins are parallel but in opposite directions, the atom is antiferromagnetic. When the spins are antiparallel but unequal in magnitude and do not cancel each other, the atom is ferromagnetic.
-Mn has 5 electrons in the d-subshell which makes its orbital half-filled. So it has maximum magnetic moment among 3d elements and has the value of magnetic susceptibility greater than zero.
-As the magnetic susceptibility is greater in magnitude and the electrons are aligned such that they cancel the effects of each other due to opposite directions, the characteristic magnetism shown by MnO is antiferromagnetism. It can be shown as
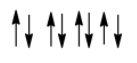
Therefore MnO is antiferromagnetic and the correct option is B.
Note: The metals like Fe, Co and Ni show ferromagnetism but when they form some compounds, their magnetic behavior changes. For example, Fe3O4 is ferromagnetic and not ferromagnetic as O also has unpaired electrons. The orientation of the electrons of Mn and O in MnO is such that they cancel their effects and so the compound is antiferromagnetic.
