Question
Question: Match the column Column I (General formula) ...
Match the column Column I (General formula)
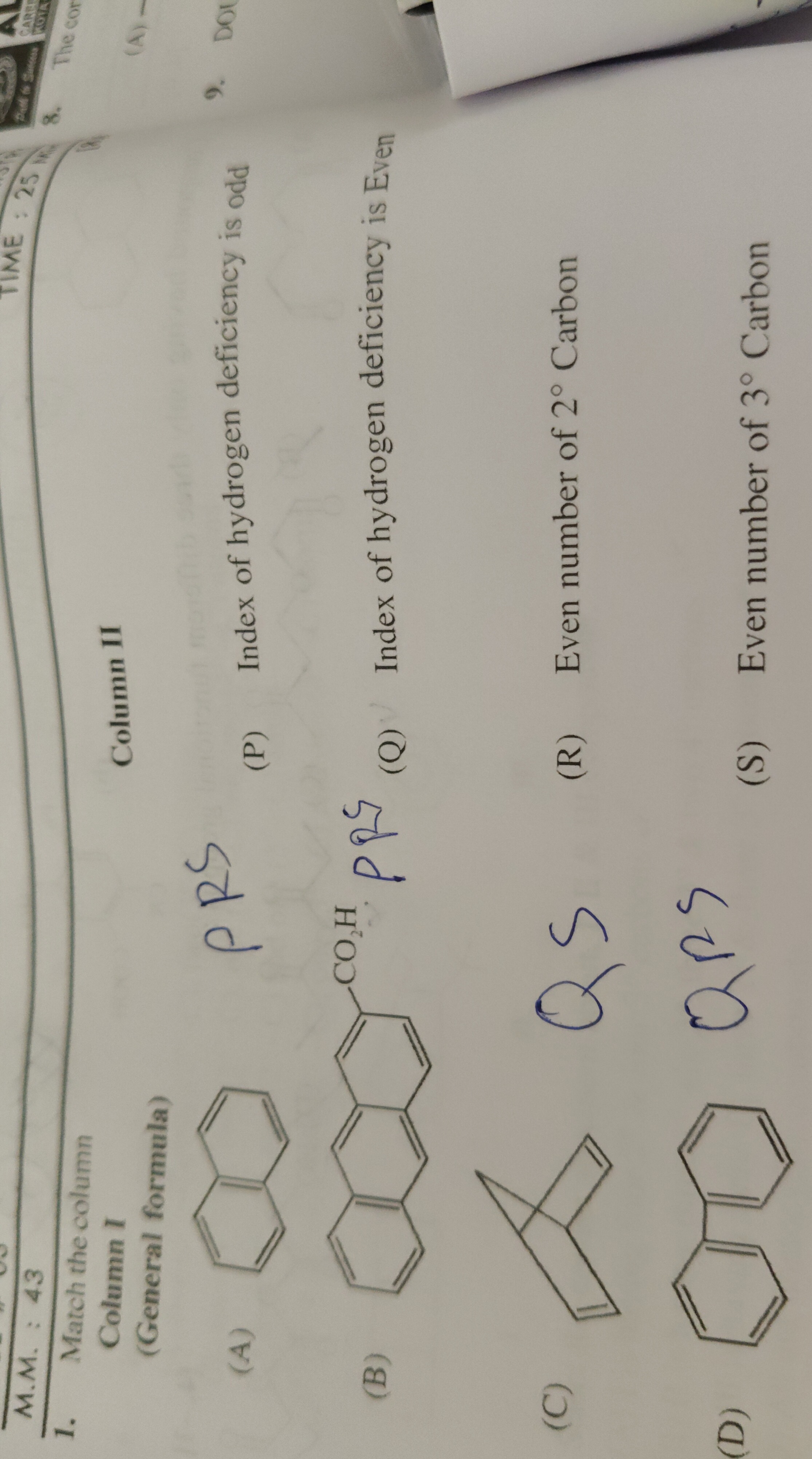
Naphthalene
Anthracene-9-carboxylic acid
Bicyclo[2.2.1]hept-2-ene (Norbornene)
Biphenyl
A-PRS, B-PR, C-PS, D-QRS
Solution
To solve this problem, we need to analyze each given organic compound for its Index of Hydrogen Deficiency (IHD), the number of secondary (2°) carbon atoms, and the number of tertiary (3°) carbon atoms.
IHD Calculation Formula:
For a compound with formula CcHhNnOoXx (where X is a halogen), the IHD is given by:
IHD=c−2h+2n−2x+1
For hydrocarbons (CcHh), it simplifies to:
IHD=c−2h+1 or IHD=22c+2−h
Carbon Classification:
- Primary (1°): Carbon atom bonded to one other carbon atom.
- Secondary (2°): Carbon atom bonded to two other carbon atoms.
- Tertiary (3°): Carbon atom bonded to three other carbon atoms.
- Quaternary (4°): Carbon atom bonded to four other carbon atoms.
Let's analyze each compound:
(A) Naphthalene
- Structure: Two fused benzene rings.
- Molecular Formula: C₁₀H₈
- IHD: IHD=22(10)+2−8=222−8=214=7.
Since IHD is 7 (odd), it matches (P). - Carbon Classification:
Naphthalene has 10 carbon atoms. The two carbons at the fusion points are bonded to 4 other carbons (4°). The remaining 8 carbons are bonded to 2 other carbons within the ring and 1 hydrogen atom each. Therefore, these 8 carbons are 2°.- Number of 2° carbons = 8 (Even) ⟹ Matches (R).
- Number of 3° carbons = 0 (Even) ⟹ Matches (S).
- Conclusion for (A): (P), (R), (S)
(B) Anthracene-9-carboxylic acid
- Structure: Anthracene (three fused benzene rings) with a -COOH group attached at position 9.
- Molecular Formula: C₁₅H₁₀O₂ (Anthracene is C₁₄H₁₀. Replacing one H with -COOH gives C₁₄H₉COOH, so C₁₅H₁₀O₂)
- IHD: For C15H10O2, oxygen atoms do not affect the IHD calculation for hydrocarbons.
IHD=22(15)+2−10=230+2−10=222=11.
Since IHD is 11 (odd), it matches (P). - Carbon Classification:
In anthracene (C14H10), there are:- 4 quaternary (4°) carbons (at the fusion points).
- 2 tertiary (3°) carbons (at positions 9 and 10, each bonded to 3 other carbons and 1 H).
- 8 secondary (2°) carbons (in the outer rings, each bonded to 2 other carbons and 1 H).
When the -COOH group is attached at position 9, the carbon at position 9 (which was 3°) becomes 4° (bonded to 3 ring carbons and the carbon of -COOH). The other carbons remain unchanged. - Number of 2° carbons = 8 (Even) ⟹ Matches (R).
- Number of 3° carbons = 1 (C10) (Odd) ⟹ Does NOT match (S).
- Conclusion for (B): (P), (R)
(C) Bicyclo[2.2.1]hept-2-ene (Norbornene)
- Structure:
(Note: C2=C3 is a double bond) - Molecular Formula: C₇H₁₀ (7 carbons, 2 rings, 1 double bond)
- IHD: IHD=number of rings+number of double bonds=2+1=3.
Since IHD is 3 (odd), it matches (P). - Carbon Classification:
- C1 (bridgehead): Bonded to C2, C6, C7. It has 1 H. ⟹ 3° carbon.
- C4 (bridgehead): Bonded to C3, C5, C7. It has 1 H. ⟹ 3° carbon.
- C2 (alkene): Bonded to C1, C3. It has 1 H. ⟹ 2° carbon.
- C3 (alkene): Bonded to C2, C4. It has 1 H. ⟹ 2° carbon.
- C5 (CH₂): Bonded to C4, C6. It has 2 H. ⟹ 2° carbon.
- C6 (CH₂): Bonded to C1, C5. It has 2 H. ⟹ 2° carbon.
- C7 (CH₂, bridge): Bonded to C1, C4. It has 2 H. ⟹ 2° carbon.
- Number of 2° carbons = 5 (Odd) ⟹ Does NOT match (R).
- Number of 3° carbons = 2 (Even) ⟹ Matches (S).
- Conclusion for (C): (P), (S)
(D) Biphenyl
- Structure: Two benzene rings directly bonded by a single bond.
- Molecular Formula: C₁₂H₁₀
- IHD: IHD=22(12)+2−10=224+2−10=216=8.
Since IHD is 8 (even), it matches (Q). - Carbon Classification:
Each benzene ring has 6 carbons. When two rings are joined, one carbon from each ring is bonded to the other ring.- The two carbons directly involved in the inter-ring bond (one from each ring) are each bonded to 2 carbons within their own ring and 1 carbon of the other ring. Thus, these two carbons are 3°.
- The remaining 5 carbons in each ring (total 10 carbons) are each bonded to 2 carbons within their own ring and 1 hydrogen. Thus, these 10 carbons are 2°.
- Number of 2° carbons = 10 (Even) ⟹ Matches (R).
- Number of 3° carbons = 2 (Even) ⟹ Matches (S).
- Conclusion for (D): (Q), (R), (S)
Final Match:
- (A) → (P), (R), (S)
- (B) → (P), (R)
- (C) → (P), (S)
- (D) → (Q), (R), (S)
The question asks to match the column, and the format implies listing all correct matches for each item in Column I.
