Question
Question: The light of wavelength $\lambda$ incident on the surface of metal having work function $\phi$ emits...
The light of wavelength λ incident on the surface of metal having work function ϕ emits the electrons. The maximum velocity of emitted electrons is (c = velocity of light, m = mass of electron, h = Planck's constant)
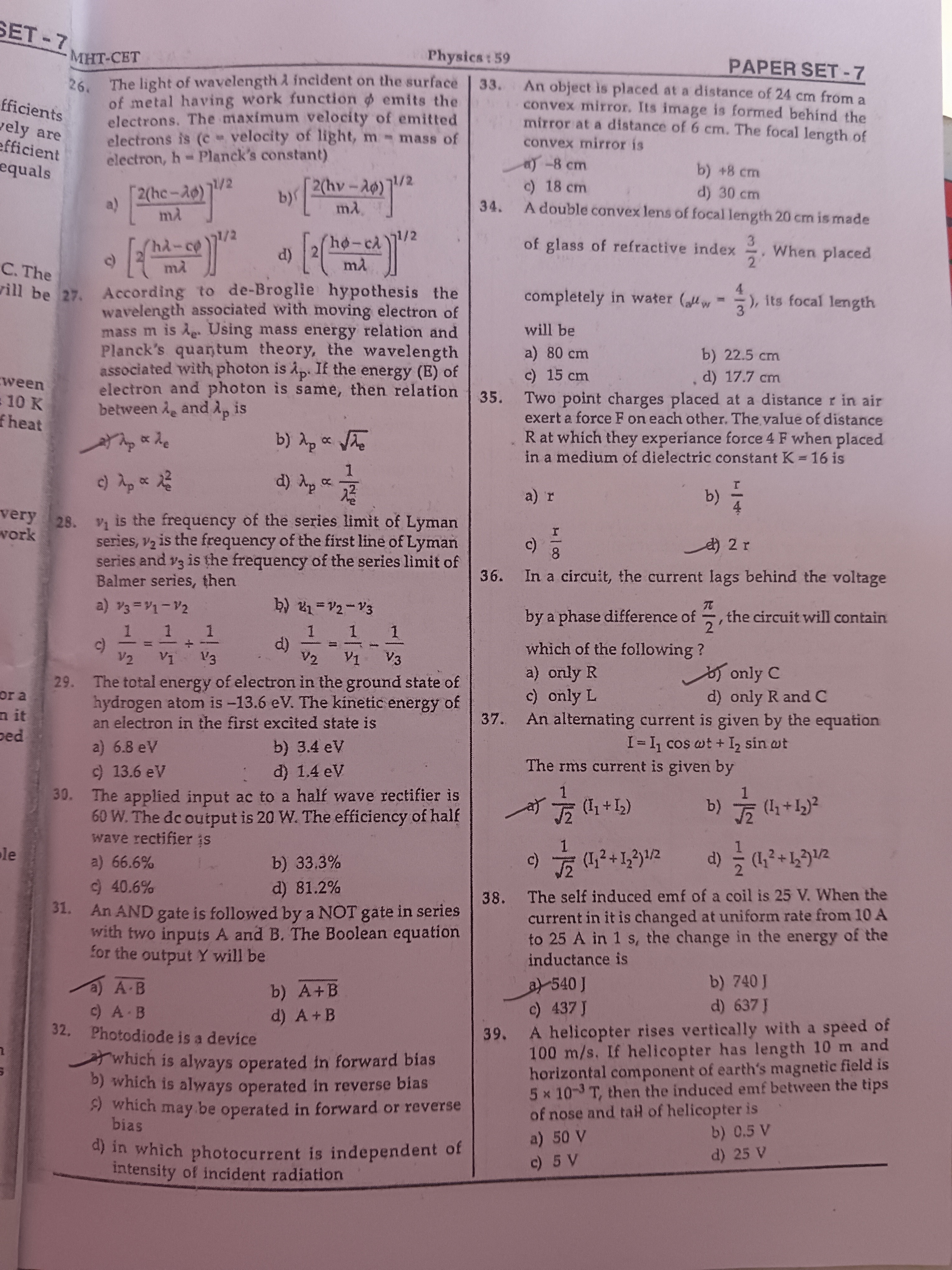
A
[mλ2(hc−λϕ)]1/2
B
[mλ2(hν−λϕ)]1/2
C
[mλhλ−cϕ]1/2
D
[2mλhϕ−cλ]1/2
Answer
[mλ2(hc−λϕ)]1/2
Explanation
Solution
Energy balance: K.E. max = h c/λ − φ Velocity: v = √[2K.E./m] = √[(2(hc/λ − φ))/m] = √[(2(hc − φλ))/(mλ)]
