Question
Question: \[MgO\] has \[NaCl\] type structure and \[TiCl\] has \[CsCl\] type? (\[MgO\] and \[TiCl\])...
MgO has NaCl type structure and TiCl has CsCl type? (MgO and TiCl)
Solution
If we analyze the lattice structure of Sodium Chloride (NaCl) and, the formula unit of NaCl (Sodium Chloride) displays a 1:1 ratio. Also, the formula unit of cesium chloride displays 1:1 ratio.
Complete step by step answer:
Given:
MgO has NaCl type structure and TiCl has CsCl type structure of ions.
Let us first understand the concept of coordination number.
The coordination number is the number of ions that immediately surround an ion of the opposite charge within a crystal lattice.
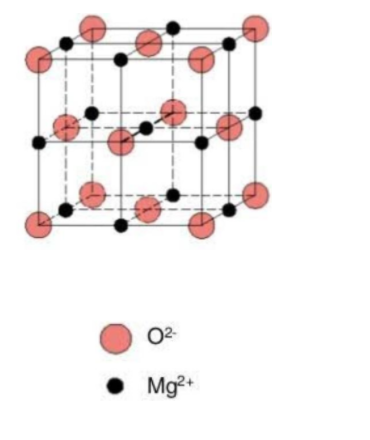
Let us now draw the similarity of MgO and NaCl structure.
In sodium chloride(NaCl)structure, the chloride ions are arranged in a face centered arrangement that is chloride ions are present at the corners and face centers of the unit cell. Therefore,
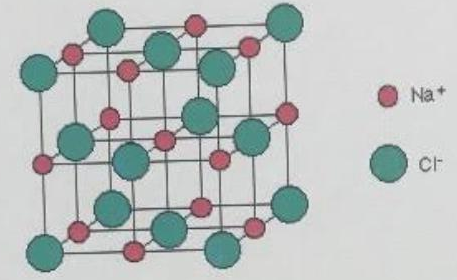
Totalnumberofchlorideionsinaunitcell=81×8+21×6
=4 [81 is the combination for the corners while 21 is combination
for face centers.]
Thus, we take 4 chlorides ions and 4 Na+ ions (present at the body center and the edge center) to balance each other. Hence, we can easily analyze that Na+ and Cl− are coordinated to 6 other atoms. The coordination number for Mg2+ and O2−in MgO is also 6 for each ion. Thus, MgO has NaCl type structure.
Similarly, in cesium chloride crystal, the cesium ion occupies center
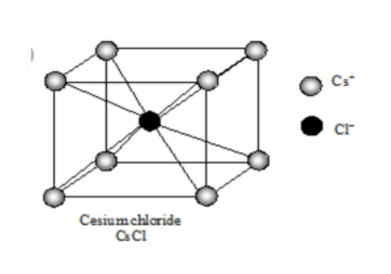
while the chloride ion occupies each corner of the cube. Thus, the
coordination of both is 8. In TiCl as well Ti+and Cl−contain coordination
number ‘8’. So CsCl and TiCl have the same structure of ions.
Note:
Students must find it very interesting that often the color of a compound is affected by the specific materials coordinated to that central ion.
