Question
Question: Methyl orange (an acid-base indicator) can be prepared by following a sequence of reactions. What ...
Methyl orange (an acid-base indicator) can be prepared by following a sequence of reactions.
What would be the structure of methyl orange?
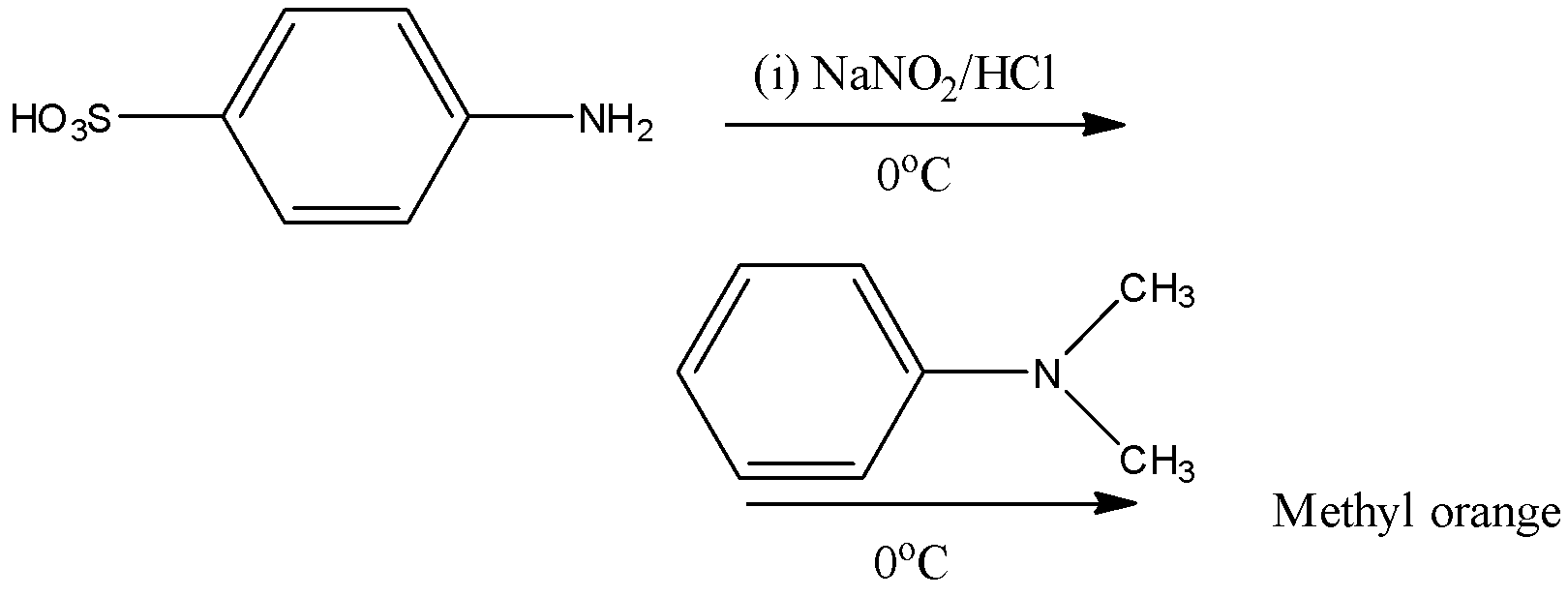
Solution
Methyl-orange is an indicator used in acid-base titration. The pH of the methyl orange indicator is 3−5. Methyl orange forms a red colouration in acidic medium but it forms a yellow colouration in basic medium. Means in acid-base titration reaction the color of the solution changed from red to yellow color.
Complete answer:
Methyl orange is going to be prepared in two steps.
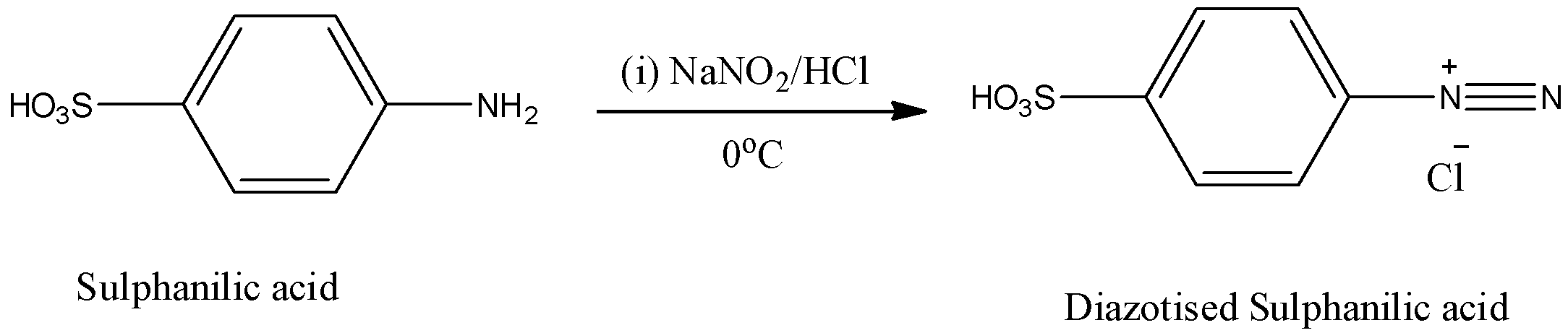
Step-1: Sulphanilic acid reacts with sodium nitrate in presence of hydrochloric acid and forms diazotized sulfanilic acid at 0oC.
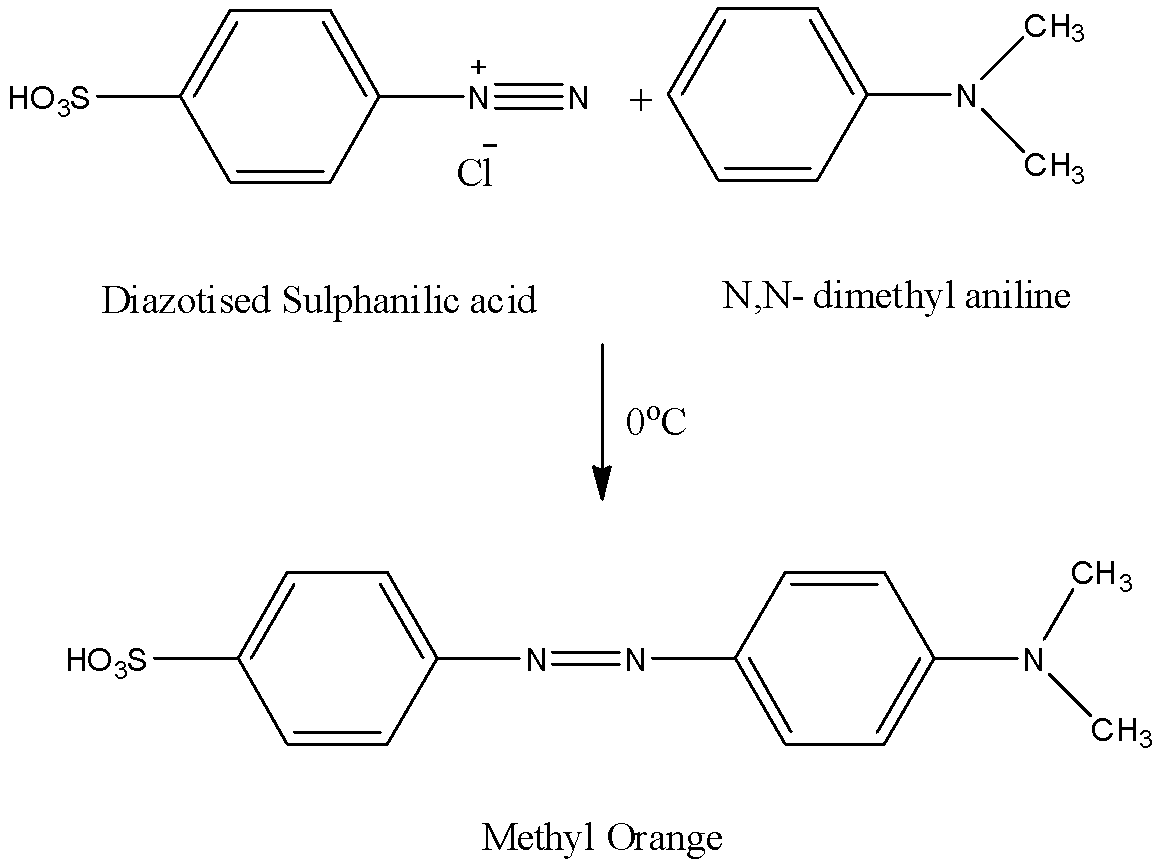
Step-2: The formed diazotized sulfanilic acid reacts with N,N-dimethyl aniline and forms methyl orange as the product.
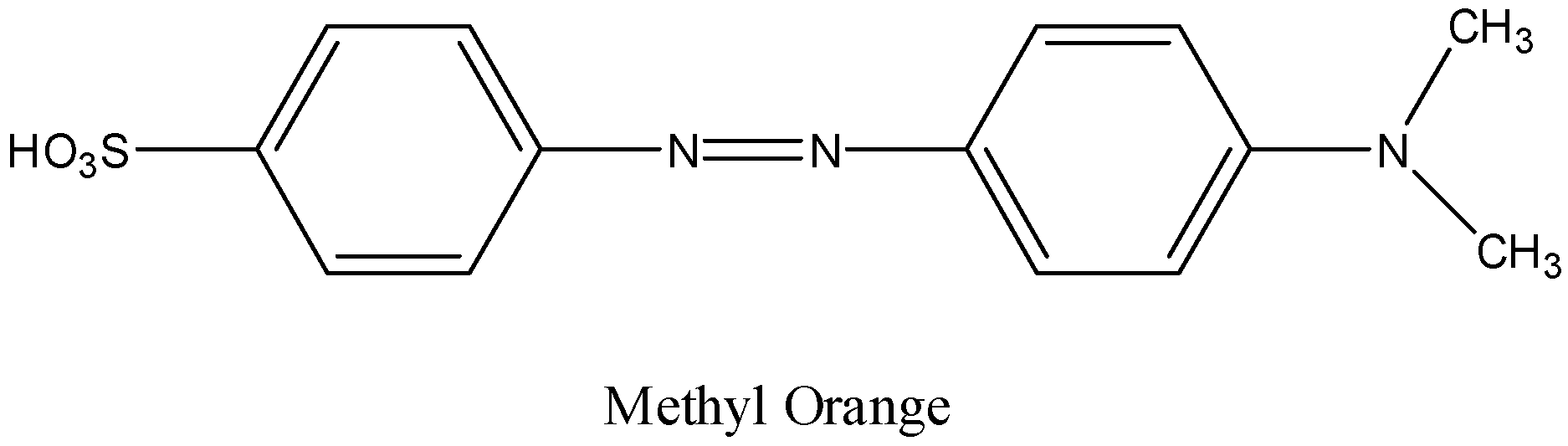
Therefore the structure of methyl orange is as follows.
Additional information:
When methyl orange reacts with acid, one of the central nitrogen atoms gets reduced and turns red in color.
The reaction of methyl orange with acid is as follows.
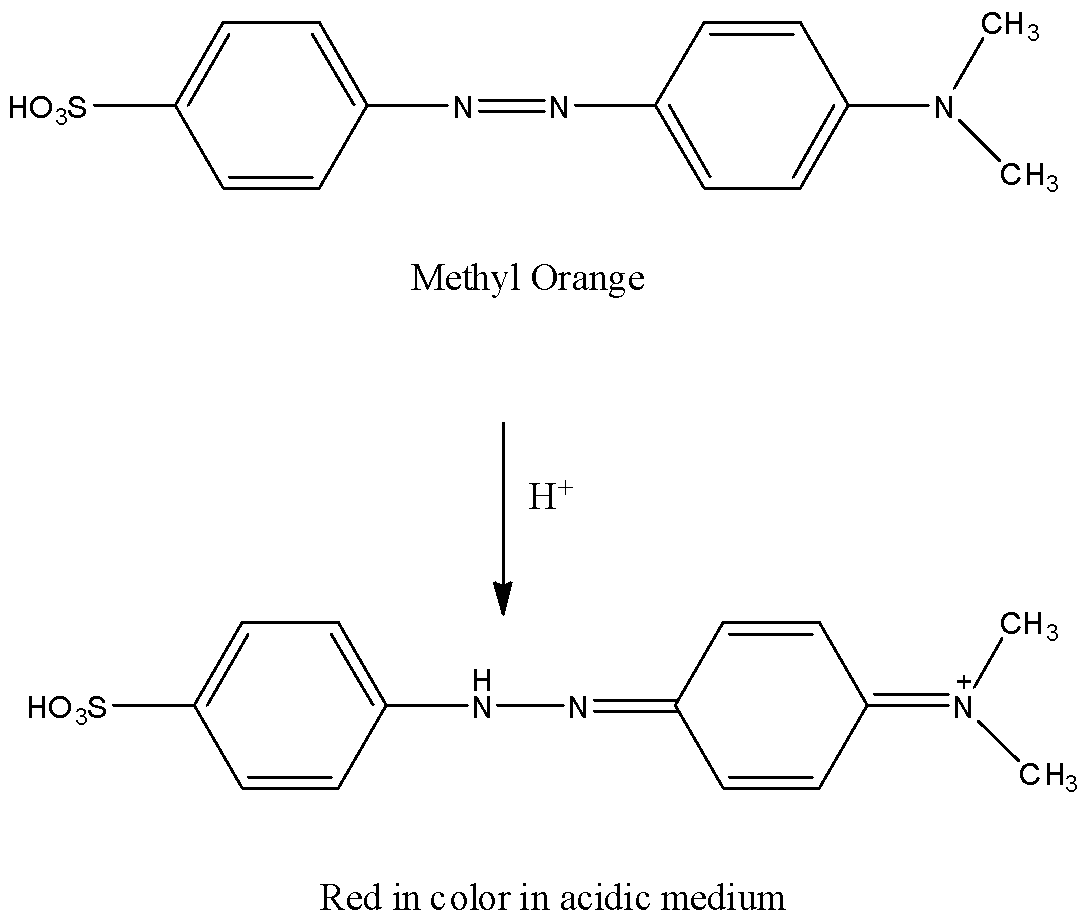
By adding a base the red color changes to yellow.
The reaction is as follows.
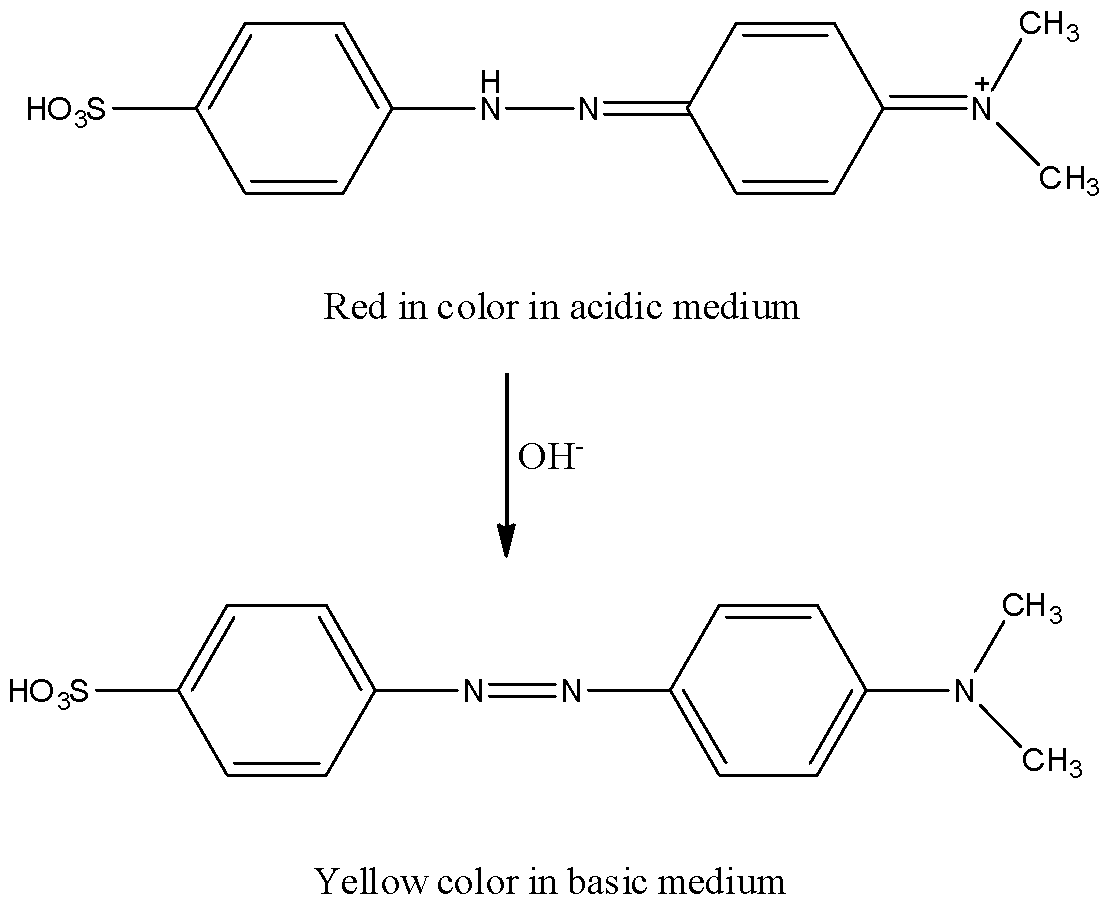
Methylorange is very rarely used in textile applications because it is sensitive to acids.
Methyl orange is a strongly colored compound and used in dyeing and in printing textiles.
Note:
Methyl orange absorbs light in the visible range of the electromagnetic spectrum and shows the color change. Methyl orange contains an extended conjugation system of delocalized electrons so it is called chromophore. Chromophore gives color to the compounds.
