Question
Question: Methyl alcohol when reacted with \(HI\) forms methyl iodide as a product. \(A.\) True \(B.\) Fal...
Methyl alcohol when reacted with HI forms methyl iodide as a product.
A. True
B. False
Solution
Methyl alcohol when reacting with HI it undergoes a substitution reaction ( i.e. alcohol (−OH) group is substituted by iodide). The reaction proceeds through the SN2 mechanism.
Complete answer:
As we know it is a substitution reaction in which alcohol group of methyl alcohol is substituted by iodide of HI and forms methyl iodide as a product. Here is the mechanism of this reaction, Methyl alcohol reacts to form alkyl halides under acidic conditions by an SN2 mechanism.
In this reaction the function of HI acid is to produce a protonated alcohol. The halide ions(I−) then displace a molecule of water from carbon, this produces a methyl iodide.
Here is the reaction involved in this mechanism,
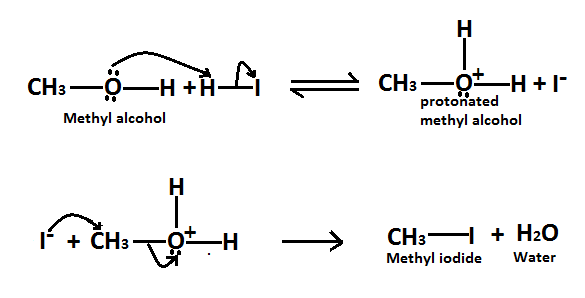
Therefore it is true when methyl alcohol reacts with HI to form methyl iodide as a product. So the correct option is A.
Additional information: As we see above methyl iodide are prepared by treatment of methyl alcohol with hydroiodic acid. It can also be prepared by heating alcohols with sodium or potassium iodide in 95% phosphoric acid. The reaction given below represents this process.
CH3OH+KI95%H3PO3CH3I+KI
Methyl alcohol: Methyl alcohol is also known as methanol, the chemical formula of methyl alcohol is (CH3OH) . It is light, volatile and flammable liquid.
Note: It is to be noted that secondary and tertiary alcohol react with methyl iodide to form alkyl halides by SN1 reaction with protonated alcohol acting as the substrate whereas the primary alcohol react with methyl alcohol to alkyl halides by SN2 reaction. The order of reactivity of alcohol with halide is as follows tertiary alcohol react faster than secondary alcohol and secondary alcohol react faster than primary alcohols
The order of reactivity of alcohols is 3∘>2∘>1∘ .
