Question
Question: Methane burns readily in air. Write the balanced chemical equation for it. A. \(C{H_4} + {20_2} \t...
Methane burns readily in air. Write the balanced chemical equation for it.
A. CH4+202→CO2+2H2O
B. CH4+302→CO2+2H2O
C. CH4+502→3CO2+2H2O
D.CH4+702→2CO2+2H2O
Solution
Combustion is a process in which compound burns in sufficient amounts of oxygen. Generally, carbon dioxide gas produced during combustion.
Step by step answer: Alkane is organic compound having general formula CnH2n+2.
If n=1 then CH2×1+2=CH4 Methane. Methane is the first member of alkane.
Since, organic compounds contain carbon and hydrogen as main elements therefore they produce carbon di-oxide and water on combustion.
Methane also produces CO2 and water on heating with oxygen or air CH4 completely oxidized by oxygen.
CH4(g)202(g)→CO2(g)+2H2O(1)
ΔH=−890KJmole−1
Negative sign represents the exothermic reaction in which heat is evolved during reaction.
Therefore, from the above explanation the correct option is (A) CH4+202→CO2+2H2O.
Additional Information: Methane is simplest alkane.
It is the main constituent of natural gas.
It is group14hydride. Methane abundantly present on earth.
Therefore, it is an economical fuel.
It is a gaseous compound so strong it processes technical challenge.
It has a tetrahedral structure. Four H-atoms are present at vertices of tetrahedron. The C-atom in methane is sp3 hybridized.
4sp3 hybrid orbitals of C-atoms form covalent bond with an orbital of 4 H-atoms.
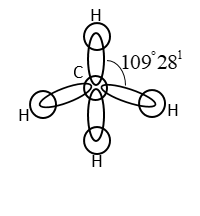
Note: Methane requires sufficient air. During incomplete combustion of alkane with insufficient amount of air carbon black is formed.
This is used in manufacture of ink, printer ink black pigments etc
