Question
Question: Arrange following in correct order of acidic strength...
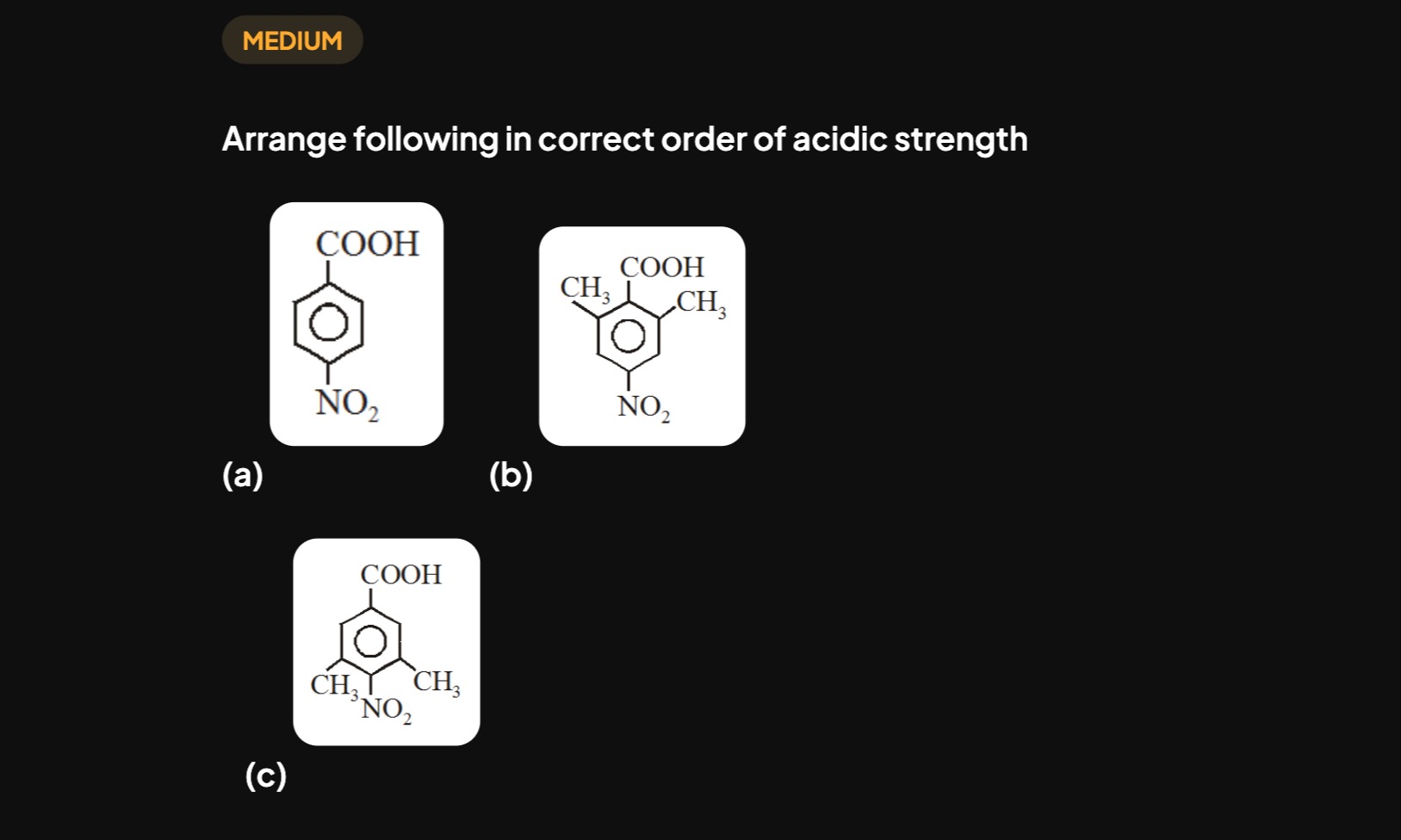
Arrange following in correct order of acidic strength
(a) > (b) = (c)
Solution
The acidic strength of carboxylic acids is determined by the stability of their conjugate bases. Electron-withdrawing groups (EWGs) stabilize the carboxylate anion, increasing acidity, while electron-donating groups (EDGs) destabilize it, decreasing acidity.
Compound (a) has a para-nitro group (-NO2), a strong EWG, which significantly stabilizes the carboxylate anion.
Compounds (b) and (c) are identical (2,6-dimethyl-4-nitrobenzoic acid). They possess both the EWG (-NO2) and two EDGs (-CH3). The electron-donating effect of the methyl groups destabilizes the carboxylate anion, reducing its acidity compared to compound (a). Therefore, (a) is the strongest acid, and (b) and (c) have equal, lower acidity.
The order is: (a) > (b) = (c).
