Question
Question: The vapour density of a sample of $N_2O_4$ gas is 35. What percent of $N_2O_4$ molecules are dissoci...
The vapour density of a sample of N2O4 gas is 35. What percent of N2O4 molecules are dissociated into NO2?
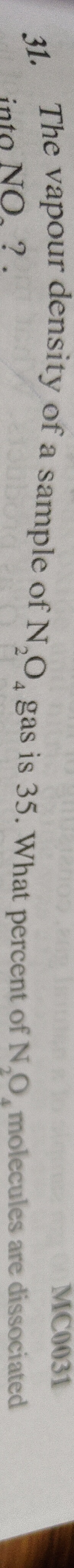
31.43%
Solution
The problem asks for the percentage dissociation of N2O4 molecules into NO2, given the observed vapour density of the gas sample.
1. Write the dissociation reaction: The dissociation of dinitrogen tetroxide (N2O4) into nitrogen dioxide (NO2) is represented as: N2O4(g)⇌2NO2(g)
2. Calculate the theoretical molecular weight of N2O4: Molar mass of Nitrogen (N) = 14 g/mol Molar mass of Oxygen (O) = 16 g/mol Theoretical molecular weight of N2O4=(2×Molar mass of N)+(4×Molar mass of O) =(2×14)+(4×16) =28+64=92 g/mol
3. Calculate the theoretical vapour density (D): Theoretical vapour density (D) is half of the theoretical molecular weight. D=2Theoretical Molecular Weight=292=46
4. Identify the observed vapour density (d): The problem states that the vapour density of the sample is 35. So, observed vapour density, d=35.
5. Determine the number of moles of products from one mole of reactant (n): From the balanced chemical equation N2O4(g)⇌2NO2(g), one mole of N2O4 dissociates to form two moles of NO2. Therefore, n=2.
6. Apply the formula for the degree of dissociation (α): The degree of dissociation (α) is related to the theoretical vapour density (D), observed vapour density (d), and the number of moles of products formed from one mole of reactant (n) by the formula: α=d(n−1)D−d
Substitute the values: D=46 d=35 n=2
α=35(2−1)46−35 α=35(1)11 α=3511
7. Calculate the percentage dissociation: α≈0.3142857 Percentage dissociation =α×100 Percentage dissociation =0.3142857×100=31.42857%
Rounding to two decimal places, the percentage dissociation is 31.43%.
The final answer is 31.43.
Explanation of the solution: The dissociation of N2O4 to NO2 is N2O4⇌2NO2.
- Calculate theoretical molar mass of N2O4: 92 g/mol.
- Calculate theoretical vapor density (D): 92/2=46.
- Given observed vapor density (d): 35.
- Use the formula for degree of dissociation (α): α=d(n−1)D−d, where n=2 (moles of products from 1 mole of reactant).
- Substitute values: α=35(2−1)46−35=3511.
- Convert to percentage: 3511×100≈31.43%.
