Question
Question: Maximum number of isomers for an alkene with molecular formula, \({{\text{C}}_{\text{4}}}{{\text{H...
Maximum number of isomers for an alkene with molecular formula,
C4H8 are,
A. 5
B. 4
C. 3
D. 2
Solution
The molecules having the same molecular formula but different arrangement of atoms are known as isomers. We will write the possible structure form the given molecular formula. Then we will determine the relation in structures that differ on the basis of position of unsaturated bond or parent chain or having different geometry.
Complete Step by step answer: From the given molecular formula we will write the possible structures for the molecule as follows:
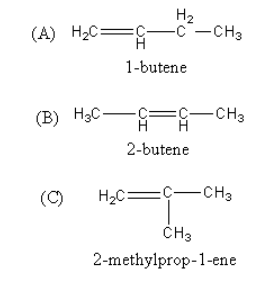
In structure A and B only the position of the double bond is different, so the A and B are known as position isomers. In A and C, the parent chain is different but the total carbon number is the same so, the A and C are the chain isomers. Similarly B and C are also chain isomers.
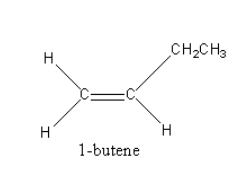
The structure of 1−butene is as follows:
Each carbon of double bond of 2−butene has two different substituents (one hydrogen and one methyl) so, it can show geometrical isomerism.
The structures of 2−butene is as follows:
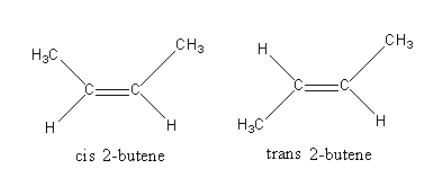
The isomer in which same priority group are on same side are known as cis isomer and the isomer in which same priority groups are on different side are known as trans isomer.
We can also draw two cyclic structure also which is as follows:
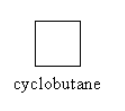
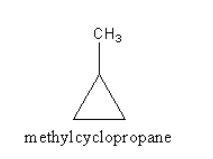
So, the maximum number of isomers for an alkene with molecular formula, C4H8 are five. Which are 1−butene, 2−butene, 2−methyl prop−1−ene, cyclobutane and methyl cyclopropane.
Therefore, option (A) 5 is correct.
Note: By the general formula of hydrocarbon we will decide that the give formula is representing the alkane, alkene or alkyne. The general formula of alkene is, CnH2n .Where, n is the number of carbon atoms. Cis and trans 2−butene both shows the same molecule. Each carbon of double bonded carbons has two groups. If the both double bonded carbons have different substituents then the substituents can have different arrangements. The isomers produced by different arrangements of substituents around double bonds are known as geometrical isomers.
