Question
Question: Maximum number of electrons present in \[{\text{Ni}}\] for which \(\left| {\text{m}} \right| = 1\), ...
Maximum number of electrons present in Ni for which ∣m∣=1, is
A) 11
B) 8
C) 12
D) 14
Solution
We know that an atom consists of electrons. The electrons are small negatively charged particles that move in an orbit around the nucleus. The electrons revolve around the nucleus. A set of numbers which is used to describe the position and movement of an electron in an atom is known as quantum number. There are four quantum numbers namely, principal quantum number, azimuthal quantum number, magnetic quantum number and spin quantum number.
Complete step-by-step answer:
There are four types of quantum numbers:
Principal quantum number: Principal quantum number is designated by n. The principal quantum number designates the shell of the atom. Principal quantum number ranges from 1, 2, 3, 4,….
Azimuthal quantum number: Azimuthal quantum number is designated by l. The azimuthal quantum number designates the subshell of the atom. If l=0 then it is s-subshell, if l=1 then it is p-subshell, if l=2 then it is d-subshell and if l=3 then it is f-subshell.
Magnetic quantum number: Magnetic quantum number is designated by m. The magnetic quantum number designates the total number of orbitals in the subshell and their orientations. The values of magnetic quantum number range between +l to −l.
Spin quantum number: Spin quantum number is designated by s. The spin quantum number designates the spin of the atom. Its values are +1/2 and −1/2.
We are given that the magnetic quantum number is 1.
The magnetic quantum number is always less than or equal to the azimuthal quantum number. Thus, the azimuthal quantum number is less than or equal to 1.
The principal quantum number is always greater than the azimuthal quantum number. Thus, the principal quantum number is greater than or equal to 2.
The atomic number of nickel is 28. The electronic configuration of nickel is as follows:
1s22s22p63s23p64s23d8
The principal quantum number must be greater than or equal to 2. Thus, the acceptable atomic orbitals are as follows:
2s 2p 3s 3p 4s 3d
The azimuthal quantum number must be greater than or equal to 1. Thus, the acceptable atomic orbitals are as follows:
2p 3p 3d
Thus, we have to calculate the maximum number of electrons having ∣m∣=1 for the electronic configuration of nickel is as follows:
2p63p63d8
We are given that ∣m∣=1. Thus, the magnetic quantum numbers can be +1 and −1. Thus, for 2p 3p 3d
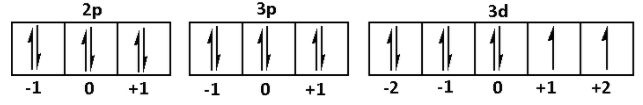
From the figure, e maximum number of electrons having ∣m∣=1 are 11.
Thus, maximum number of electrons present in Ni for which ∣m∣=1 is 11.
Thus, the correct answer is option ‘A’.
Note: Remember for p-orbital the values of magnetic quantum number are −1,0,+1 and for the d-orbital the values of magnetic quantum number are −2,−1,0,+1,+2. We are given that ∣m∣=1. This indicates the value of magnetic quantum numbers can be positive or negative.
