Question
Question: Maximum number of atoms lie in one plane for the molecule. ...
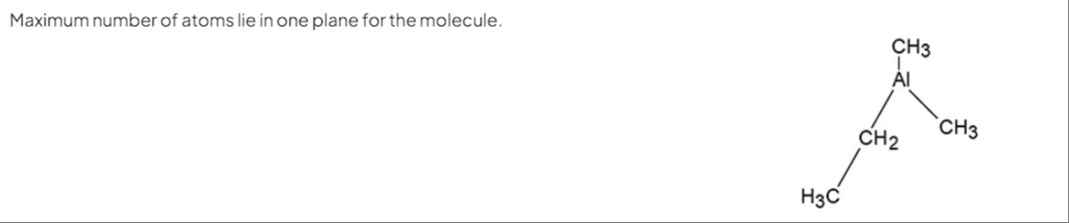
Maximum number of atoms lie in one plane for the molecule.
8
Solution
The molecule is diethylmethylaluminium, Al(CH3)2(CH2CH3). The aluminium atom is bonded to two methyl groups and one ethyl group. The aluminium atom is sp2 hybridized, and the three bonds from Al to the carbon atoms of the alkyl groups lie in a plane. Let's call the carbon atoms directly bonded to Al as C1 (from first CH3), C2 (from second CH3), and C3 (from CH2 of ethyl group). The atoms Al, C1, C2, and C3 lie in the same plane. This gives 4 atoms.
Now consider the alkyl groups. Each methyl group (CH3) is attached to C1 and C2. The carbon atom and one hydrogen atom of a methyl group can lie in the plane containing the Al-C bond. So, from the CH3 group on C1, we can have C1 and one H in the plane. Similarly, from the CH3 group on C2, we can have C2 and one H in the plane.
The ethyl group is -CH2CH3, attached to C3. Let C4 be the carbon atom of the CH3 part of the ethyl group. The atoms Al, C3, and C4 can be in the same plane by rotating around the Al-C3 bond. Since Al, C1, C2, C3 are in a plane, we can orient the ethyl group such that C4 is also in this plane. So, Al, C1, C2, C3, C4 can be in the same plane. This gives 5 carbon atoms and Al, total 6 atoms. However, Al, C1, C2, C3 are in a plane. We can orient the ethyl group such that C4 is in this plane. So, Al, C1, C2, C3, C4 are in the plane. (5 atoms).
Now let's consider the hydrogen atoms. Plane contains Al, C1, C2, C3. (4 atoms) From CH3 on C1: C1 and one H. (2 atoms). From CH3 on C2: C2 and one H. (2 atoms). From CH2CH3 on C3: The CH2 group has C3 bonded to Al, C4, and two H atoms (H3a, H3b). We can orient the ethyl group such that the C3−C4 bond is in the plane. So C4 is in the plane. The CH3 group on C4 has C4 bonded to C3 and three H atoms. We can orient the CH3 group such that one H on C4 is in the plane.
Consider the plane containing Al, C1, C2, C3. Atoms in this plane: Al, C1, C2, C3. (4 atoms) From CH3 on C1: C1 and one H. So, Al, C1, HC1, C2, C3 are in the plane. (5 atoms) From CH3 on C2: C2 and one H. So, Al, C1, HC1, C2, HC2, C3 are in the plane. (6 atoms) From CH2CH3 on C3: C3 is in the plane. C3 is bonded to C4 and two H atoms. We can orient the ethyl group such that the C3−C4 bond is in the plane. So C4 is in the plane. Al, C1, HC1, C2, HC2, C3, C4 are in the plane. (7 atoms). Now consider the hydrogen atoms on C3 and C4. Since Al, C3, C4 are in the plane, the two H atoms on C3 are out of the plane. For the CH3 group on C4, since C3 and C4 are in the plane, we can orient the CH3 group such that one H is in the plane. So, atoms in the plane: Al, C1, HC1, C2, HC2, C3, C4, HC4. Total 1+1+1+1+1+1+1+1=8 atoms.
Let's consider a different plane. Consider the plane containing the ethyl group in its extended conformation. The atoms C3−C4 and all the hydrogen atoms on C3 and C4 cannot be in one plane. However, the atoms C3, C4, and one H from C4 can be in a plane. The atoms Al, C3, C4 can be in a plane. Consider the plane containing Al, C3, C4. We can have one H from C4 in this plane. So, Al, C3, C4, HC4 are in the plane. Now consider the methyl groups. Al is in the plane. C1 and C2 are attached to Al. Since Al, C3 are in the plane, C1 and C2 are also in the plane containing Al and C3 if Al, C1, C2, C3 are in a plane. So, the plane contains Al, C1, C2, C3, C4. And one H from C1, one H from C2, one H from C4. Total atoms = Al+C1+C2+C3+C4+HC1+HC2+HC4=1+1+1+1+1+1+1+1=8.
Let's think about the ethyl group. Can we have more atoms from the ethyl group in a plane? The atoms C3, C4, and the three H atoms on C4 form a tetrahedral arrangement around C4. The atoms C3, C4, and one H on C4 can be in a plane. The atoms C3, and the two H atoms on C3 form a part of tetrahedral arrangement around C3. The atoms Al, C3, and one H on C3 can be in a plane. Consider the plane containing Al, C3, and C4. We can have one H from C4 in this plane. So we have Al, C3, C4, HC4. (4 atoms). Since Al, C1, C2, C3 are in a plane, and Al, C3, C4 are in the same plane by rotation, the plane contains Al, C1, C2, C3, C4. (5 atoms). We can have one H from C1 in the plane, one H from C2 in the plane, and one H from C4 in the plane. Total 5+1+1+1=8 atoms.
Can we get more than 8 atoms? Consider the ethyl group in its extended conformation. The atoms C3−C4−H bond angle is approximately 109.5∘. The atoms Al−C3−C4 angle is approximately 109.5∘. The atoms Al, C3, C4 are in a plane. We can have one H from C4 in this plane. So Al, C3, C4, HC4 are in the plane. Now consider the CH2 group at C3. The C3 atom is bonded to Al, C4, H3a, H3b. Since Al, C3, C4 are in the plane, the two H atoms on C3 are out of the plane.
Let's consider the entire ethyl group in the plane, i.e., C3, C4, H3a, H3b, H4a, H4b, H4c. This is 7 atoms. Can we have Al in this plane? No, because the Al-C3 bond is roughly tetrahedral with respect to the bonds around C3. So, the entire ethyl group cannot be in a plane with Al.
Consider the plane containing Al and the entire ethyl group in an extended conformation. The atoms Al, C3, C4, and the five hydrogen atoms of the ethyl group is a total of 1+2+5=8 atoms. Let's see if we can have all atoms of the ethyl group in a plane containing Al. The ethyl group is -CH2CH3. The maximum number of atoms in a plane for ethane is 6 (all atoms in eclipsed conformation). However, here the ethyl group is attached to Al. The atoms Al, C3, C4, H3a, H3b, H4a, H4b, H4c are the atoms of the ethyl group plus Al. Total 1+2+5=8 atoms. Can we arrange them in a plane? Consider the plane containing Al, C3, C4. We can orient the CH3 on C4 such that one H is in the plane. So Al, C3, C4, HC4 are in the plane. The CH2 group at C3 is bonded to Al, C4, H3a, H3b. Since Al, C3, C4 are in the plane, the two H atoms on C3 are out of the plane.
Let's consider the plane containing Al, C3, and one H on C3. Let this be H3a. So, Al, C3, H3a are in the plane. The C3 is also bonded to C4 and H3b. Can C4 be in this plane? Yes, if the dihedral angle around Al-C3 is appropriate. Can H3b be in this plane? No, if C4 is in the plane, since the bonds around C3 are roughly tetrahedral. If Al, C3, H3a, C4 are in a plane, then from the CH3 on C4, we can have one H in the plane. So Al, C3, H3a, C4, HC4 are in the plane. (5 atoms from ethyl group and Al). Now consider the methyl groups on C1 and C2. Al is in the plane. C1 and C2 are bonded to Al. The Al-C1 and Al-C2 bonds are out of the plane containing Al, C3, H3a.
Let's go back to the plane containing Al, C1, C2, C3. And orient the alkyl groups to maximize the number of atoms in this plane. Al, C1, C2, C3 are in the plane. (4 atoms) From CH3 on C1: C1 and one H. (2 atoms). From CH3 on C2: C2 and one H. (2 atoms). From CH2CH3 on C3: C3 is in the plane. Orient such that C4 is in the plane. (1 atom). Orient CH3 on C4 such that one H is in the plane. (1 atom). Total atoms = Al+C1+C2+C3+HC1+HC2+C4+HC4=1+1+1+1+1+1+1+1=8.
Let's consider the possibility of including more hydrogen atoms from the ethyl group. Consider the plane containing the ethyl group in an extended conformation. This plane contains C3, C4, H3a, H3b, H4a (one H on C4 in this plane). Total 5 atoms from the ethyl group. Can we include Al in this plane? Yes, by rotating around the Al-C3 bond. So, Al, C3, C4, H3a, H3b, H4a are in the plane. Total 1+2+3=6 atoms from ethyl group and Al. Now consider the methyl groups. Al is in the plane. C1 and C2 are bonded to Al. The Al-C1 and Al-C2 bonds are out of the plane containing Al, C3, H3a. So, we cannot include C1 and C2 in this plane unless they happen to lie in this plane.
Let's go back to the plane containing Al, C1, C2, C3. And orient the alkyl groups. Al, C1, C2, C3 are in the plane. (4 atoms) From CH3 on C1: C1 and one H. (2 atoms) From CH3 on C2: C2 and one H. (2 atoms) From CH2CH3 on C3: C3 is in the plane. Orient such that C4 is in the plane. (1 atom). Orient CH3 on C4 such that one H is in the plane. (1 atom). Total atoms = Al+C1+C2+C3+HC1+HC2+C4+HC4=1+1+1+1+1+1+1+1=8.
Let's consider another plane. The plane containing Al, C3, C4, and the three hydrogen atoms on C4. This is not possible since the bonds around C4 are tetrahedral. Maximum number of atoms in a plane for C3−CH3 part is C3, C4, and one H on C4. (3 atoms). Or C4 and two H on C4. (3 atoms).
Let's reconsider the plane containing Al, C1, C2, C3. Atoms in this plane: Al, C1, C2, C3. (4 atoms) From CH3 on C1: C1 and one H. From CH3 on C2: C2 and one H. From CH2CH3 on C3: C3 is in the plane. C3 is bonded to C4 and two H atoms. We can orient the ethyl group such that the C3−C4 bond is in the plane. So C4 is in the plane. Now we have Al, C1, C2, C3, C4 in the plane. (5 atoms). From CH3 on C1: one H. From CH3 on C2: one H. From CH2 on C3: Since Al, C3, C4 are in the plane, the two H atoms on C3 are out of the plane. From CH3 on C4: Since C3 and C4 are in the plane, we can orient the CH3 such that one H is in the plane. So, atoms in the plane are Al, C1, C2, C3, C4, one H from C1, one H from C2, one H from C4. Total 8 atoms.
Let's think about the ethyl group again. The atoms C3, C4, and the three H atoms on C4. Can we put Al and all these atoms in a plane? No. Can we put Al, C3, C4, the two H atoms on C3, and the three H atoms on C4 in a plane? This is 1+2+5=8 atoms. This corresponds to the entire ethyl group and Al. This is possible if the ethyl group is planar and the Al-C3 bond is in that plane. However, the ethyl group is not planar.
Consider the plane containing Al, C3, and the C3−C4 bond. This plane contains Al, C3, C4. We can orient the CH3 on C4 such that one H is in the plane. Now, consider the CH2 group on C3. The C3 is bonded to Al, C4, and two H atoms. Since Al, C3, C4 are in the plane, the two H atoms on C3 are out of the plane.
Let's consider the plane containing Al and the entire ethyl group in the extended conformation. The ethyl group in extended conformation has the carbon backbone C3−C4 and the attached H atoms. The atoms C3, C4, and the two H on C3 and the three H on C4 are 7 atoms. Can we include Al in this plane? Yes, if the Al-C3 bond is in this plane. So, Al and the entire ethyl group in extended conformation can be in a plane. This is 1+7=8 atoms. Now consider the methyl groups. Al is in the plane. C1 and C2 are bonded to Al. The Al-C1 and Al-C2 bonds are out of this plane. So, we cannot include any atoms from the methyl groups in this plane, except for Al.
So, we found a plane with 8 atoms: Al and all atoms of the ethyl group in extended conformation. Let's check if the plane containing Al, C1, C2, C3, C4, HC1, HC2, HC4 is the same plane. The plane containing Al, C1, C2, C3 is the trigonal plane around Al. If the ethyl group is in extended conformation, the atoms C3, C4, and the H atoms are arranged in a certain way.
Let's assume the maximum number of atoms is 8.
Final check: Plane containing Al and the entire ethyl group in extended conformation. Atoms: Al, C3, H3a, H3b, C4, H4a, H4b, H4c. Total 8 atoms. In this plane, Al is bonded to C1 and C2. These bonds are out of the plane. So no atoms from the methyl groups (except Al) can be in this plane.
Consider the plane containing Al, C1, C2, C3. This is the trigonal plane. We can have one H from C1 and one H from C2 in this plane. So, Al, C1, HC1, C2, HC2, C3. Total 6 atoms. From the ethyl group on C3. C3 is in the plane. Can we have C4 in this plane? Yes, by rotating around Al-C3. So, Al, C1, HC1, C2, HC2, C3, C4. Total 7 atoms. From CH2 on C3, the two H are out of the plane. From CH3 on C4, since C3 and C4 are in the plane, we can have one H in the plane. So, Al, C1, HC1, C2, HC2, C3, C4, HC4. Total 8 atoms.
Let's consider the case where the ethyl group is in the plane. The atoms C3, C4, H3a, H3b, H4a, H4b, H4c are 7 atoms. If Al is also in this plane, total 8 atoms.
Consider the plane containing Al, C3, C4. We can have one H from C4 in the plane. And the two H from C3 are out of the plane. Consider the plane containing Al, C3, and one H from C3. Let's say H3a. Can C4 be in this plane? Yes, by rotation. Can the other H on C3, H3b, be in this plane? No. Can the three H on C4 be in this plane? No. Can we include C1 and C2 in this plane? No.
The maximum number of atoms in one plane is 8.
